Figure 6.
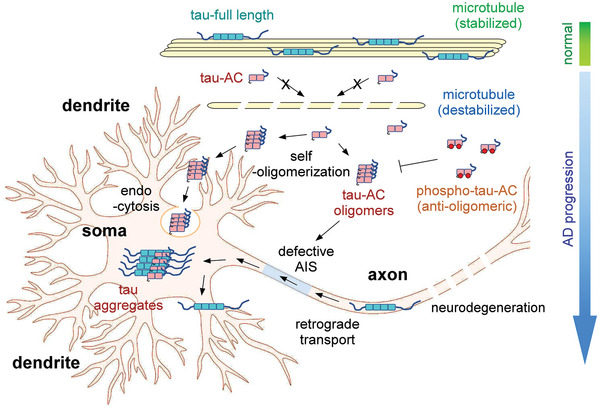
The proposed model of tau‐AC‐induced pathogenesis. Tau‐AC has a weaker binding affinity to microtubules than tau‐FL. During the onset of AD, free (unbound) tau‐AC species spontaneously oligomerize through their N‐terminal residues forming proto‐filaments. Neurons can internalize the oligomeric forms of tau‐AC through endocytosis, leading to endogenous tau aggregation. However, phospho‐mimetic tau‐AC exhibits a drastically reduced aggregation propensity, suggesting that phosphorylation may have anti‐amyloidogenic properties. The pathologic tau species are mislocalized in somatodendrites and dendritic spines due to the perturbed AIS function, ultimately causing excitotoxicity. The clinical significance of this tau fragment in various tauopathies remains to be determined. Furthermore, these findings may pave the way for the development of novel pharmacological approaches designed to inhibit tau filament formation.
