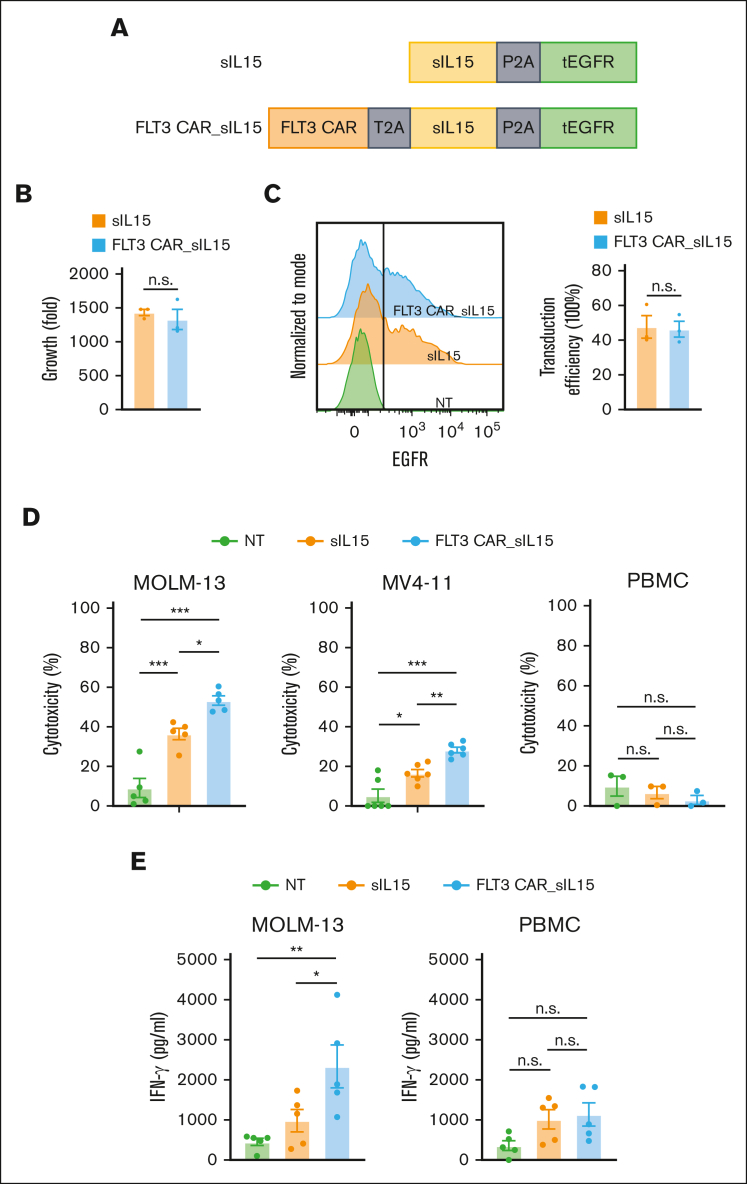
Figure 4.
FLT3 CAR_sIL-15 cord blood NK cells enhance cytotoxicity against FLT3+AML. (A) Scheme of FLT3 CAR_sIL-15 and its parental control (sIL-15) vector that expresses IL-15 but not the CAR. tEGFR is coexpressed to serve as a marker. (B) The expansion capacity of primary cord blood NK cells engineered with sIL-15 or FLT3 CAR_sIL-15 after 16 days of culture (n = 3 donors). (C) The transduction efficiencies of FLT3 CAR_sIL-15 NK cells and sIL-15 NK cells are determined based on the expression of tEGFR at the time of harvesting (day 16) (n = 3 donors). (D) The cytotoxicity of NT, sIL-15 NK, and FLT3 CAR_sIL-15 NK cells against FLT3+ AML cells (MOLM-13 or MV-411, n = 6 donors each) or FLT3-negative (FLT3−) cells (PBMC) (n = 3 donors), assessed via flow-based cytotoxicity assay at an E:T ratio of 4:1 for PBMCs and MV-411 and 2:1 for MOLM-13. (E) IFN-γ production of NT, sIL-15 NK, and FLT3 CAR_sIL-15 NK cells (n = 5 donors) against FLT3+ AML cells (MOLM-13) or FLT3− cells (PBMCs) at an E:T ratio of 4:1 for 18 hours and measured by enzyme-linked immunosorbent assay. Data are presented as mean ± SEM. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.