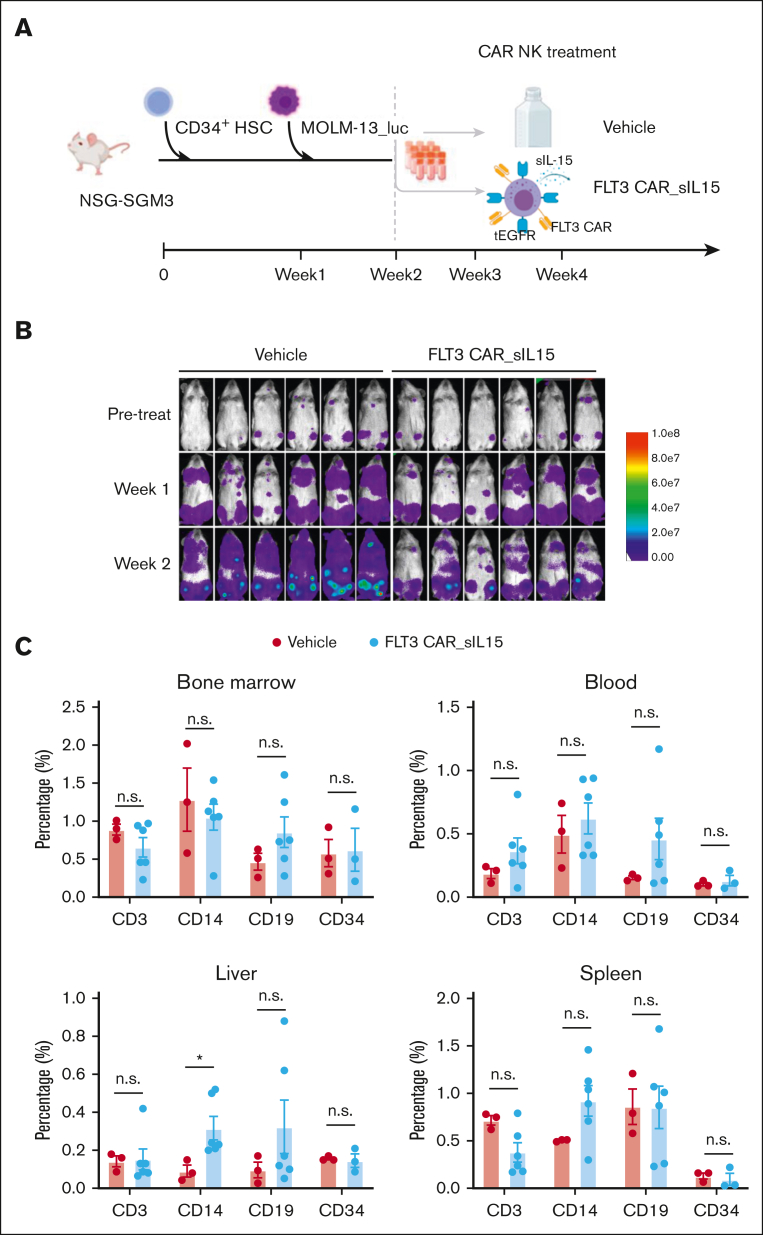
Figure 7.
Validation of FLT3 CAR_sIL-15 NK cell safety on human CD34 HSC cells in an AML mouse model. (A) Scheme of CD34+ HSC inoculation and FLT3 CAR_sIL-15 NK cell treatment. NSGS mice first received grafts of CD34+ HSCs (3 × 105 cells), followed 1 week later by receiving FLT3+ AML tumor cell grafts (5 × 105 MOLM-13_luc_GFP cells), which was followed by treatment with off-the-shelf frozen and then thawed (1 × 107 cells) FLT3 CAR_sIL-15 NK cells on days 14 and 21. Mice were euthanized on day 28 and human HSCs along with human lymphocyte subsets were isolated from the mice and subjected to Fluorescence-activated cell sorting analysis. (B) Time-lapsed luciferase images of the mice inoculated with CD34+ HSC and MOLM-13_luc_GFP AML cells and then treated with either vehicle control or FLT3 CAR_sIL-15 NK cells. (C) Fluorescence-activated cell sorting analysis of human B cells (CD19), T cells (CD3), HSCs (CD34), and monocytes (CD14) among the human CD45+ GFP− cells isolated from the BM, PB, liver, and spleen of NSGS mice that received engraftment with CD34+ HSCs (n = 3 or 6 mice per group). Percentages are calculated from cells gated on live human CD45+ GFP− single cells. Data are presented as mean ± SEM; ∗P < .05. The figure was generated with BioRender.com.