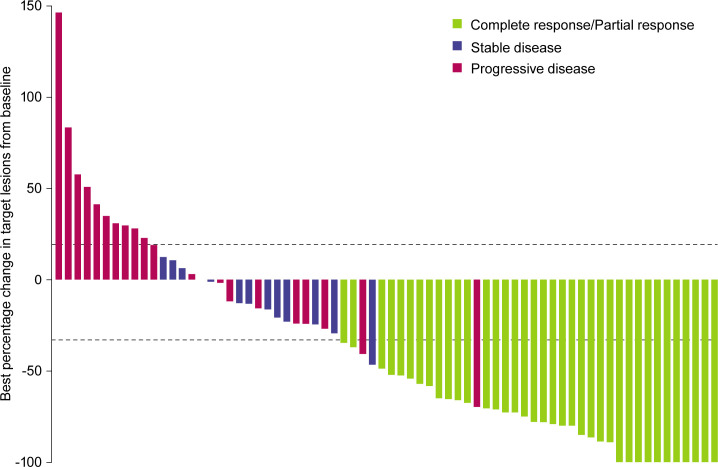
Figure 1.
mCSCC best overall response to cosibelimab monotherapy. Best percentage change in the sum of target lesion diameters from baseline for participants who underwent tumor assessment by independent central review after treatment initiation (n=70). Figure excludes participants with a best overall response of not evaluable due to no post-baseline tumor assessment (n=8), which are included as non-responders in the calculation of ORR. Horizontal dashed lines indicate RECIST V.1.1 criteria for partial response (≥30% decrease in the sum of target lesion diameters) and progressive disease (≥20% increase in target lesion diameters). mCSCC, metastatic cutaneous squamous cell carcinoma; ORR, objective response rate; RECIST V.1.1, Response Evaluation Criteria in Solid Tumors, version 1.1.