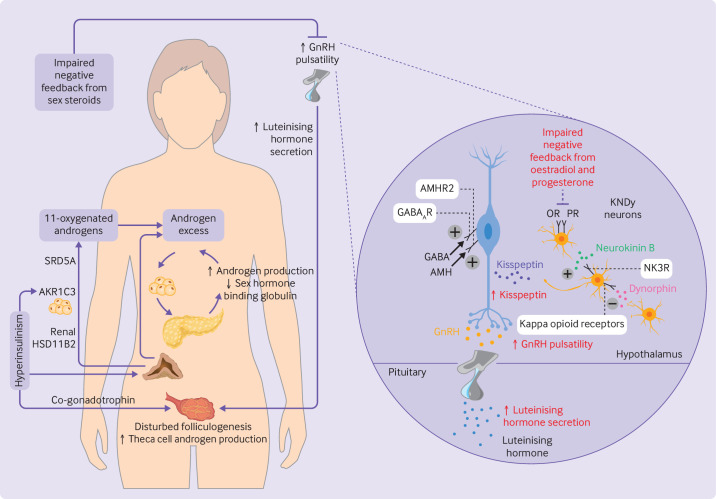
Figure 1.
Pathophysiology and neuroendocrine disruption of the hypothalamo-pituitary-gonadal axis in polycystic ovary syndrome. (Left) Increased pulsatility of gonadotrophin releasing hormone (GnRH) causes increased secretion of luteinising hormone, consequent disrupted folliculogenesis, and increased production of ovarian androgens. Adrenal androgens are also increased, including 11-oxygenated androgens which are activated peripherally by renal 11β-hydroxysteroid dehydrogenase type 2 (HSD11B2) and aldo-keto reductase 1C3 (AKR1C3) in adipocytes. Steroid-5α-reductase (SRD5A) converts 11-ketotestosterone to 11-ketodihydrotestosterone. Excess levels of androgens stimulate deposition of abdominal adipose tissue which subsequently increases insulin resistance and hyperinsulinism. Hyperinsulinism stimulates AKR1C3 activity, increases androgen production from the ovaries (by its action as a co-gonadotrophin) and adrenal cortex, reduces production of hepatic sex hormone binding globulin, and inhibits progesterone mediated negative feedback onto GnRH neurons, worsening androgen excess in a vicious cycle. (Right) Kisspeptin, neurokinin B, and dynorphin A neurons (KNDy neurons) act in a paracrine and autocrine way to regulate release of kisspeptin onto GnRH neurons and consequent GnRH pulsatility. Neurokinin B binds to neurokinin 3 receptors (NK3R) to stimulate release of kisspeptin whereas dynorphin binds to kappa opioid receptors to inhibit kisspeptin release. γ-aminobutyric acid (GABA) and anti-müllerian hormone (AMH) bind to GABAA receptors (GABAAR) and AMH receptor type 2 (AMHR2), respectively, to stimulate GnRH pulsatility. Impaired negative feedback from oestradiol and progesterone is seen at the level of the hypothalamus. Neuroendocrine abnormalities in the control of these components are shown in red. OR=oestrogen receptor; PR=progesterone receptor