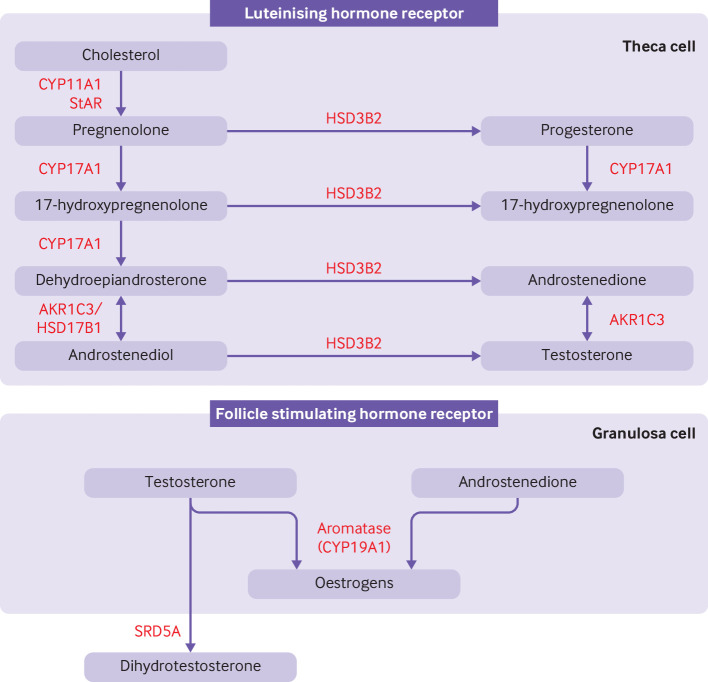
Figure 2.
Classical pathway of androgen synthesis. Luteinising hormone stimulates the classical pathway of androgen synthesis in ovarian theca cells. Cholesterol is transported to the inner mitochrondrial membrane by steroidogenic acute regulatory protein (StAR). A cleavage system of the cytochrome P450 enzyme, CYP11A1, ferrodoxin, and ferrodoxin reductase converts cholesterol to pregnenolone. Expression of CYP11A1 is stimulated by activation of the luteinising hormone receptor. Pregnenolone is transported to smooth endoplasmic reticulum where it is converted to 17-hydroxypregnenolone and subsequently to dehydroepiandrosterone by the 17-hydroxylase and 17,20-lyase subunit of the CYP17A1 enzyme, respectively. Dehydroepiandrosterone is then converted to androstenedione or androstenediol and subsequently to testosterone by a combination of 3β-hydroxysteroid dehydrogenase type II (HSD3B2) and aldo-keto reductase type 1C3 (AKR1C3). 17β-hydroxysteroid dehydrogenase 1 (HSD17B1) also catalyses the conversion of dehydroepiandrosterone to androstenediol. HSD3B2 converts pregnenolone and 17-hydroxypregnenolone to progesterone and 17-hydroxyprogesterone, respectively, which are substrates for a back door alternative pathway of androgen synthesis. Androstenedione and testosterone diffuse into granulosa cells where they are converted to oestrogens by the action of aromatase (CYP19A1), under the control of follicle stimulating hormone receptor activation. Testosterone can be converted to dihydrotestosterone by steroid 5α-reductase (SRD5A) in peripheral tissues