Extended Data Fig. 10 |. Comparison of VchTnsC and TnsC homologues from evolutionarily diverse transposons.
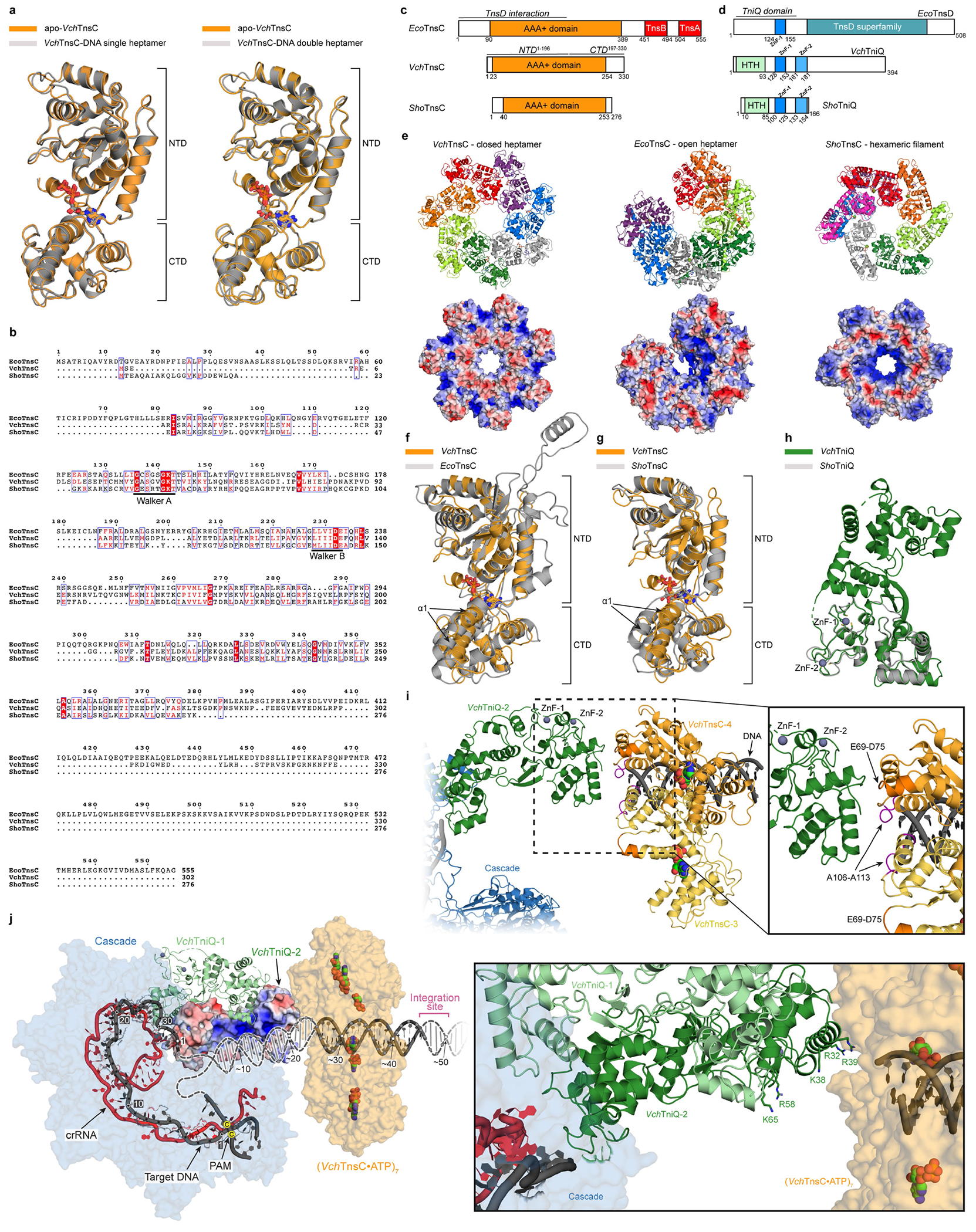
a, The structure of TnsC is highly similar between apo and DNA-bound states. Structural superposition of a monomer from the apo-TnsC and DNA-bound TnsC single heptamer structure yields a global RMSD of 0.53 Å (left); a superposition with the double heptamer yields a global RMSD of 0.64 Å (right). ATP molecules are shown as sticks, and the NTD and CTD are labelled. b, Multiple sequence alignment of EcoTnsC22 from Tn7 (non-CRISPR associated), VchTnsC from this study (type I-F CRISPR-associated), and ShoTnsC from ShCAST3,23,24 (type V-K CRISPR-associated). Residues critical for ATPase activity are indicated. Sequence identities are as follows: VchTnsC and EcoTnsC, 18.84%; VchTnsC and ShoTnsC, 22.18%; EcoTnsC and ShoTnsC, 20.83%. c, Comparison of domain organization between three TnsC homologues, with EcoTnsC regions that interact with TnsA, TnsB, and TnsD indicated4. Note the significantly shorter N- and C-termini for VchTnsC and ShoTnsC. d, Comparison of domain organization between three TniQ-family protein homologues; note that the E. coli Tn7 protein is referred to as TnsD. Zinc-finger (ZnF) motifs are highlighted. e, Comparison of overall oligomeric architecture (top) and APBS electrostatic potential70 (bottom) for DNA-bound VchTnsC•ATP (left; this study), DNA-bound EcoTnsC(A225V)•AMPPNP (centre; PDB ID: 7MCS)22 and DNA-bound ShoTnsC•ATPɣS (right; PDB ID: 7M99)23, with the C-terminal face of the pore pointing up in each oligomer. DNA was omitted for clarity and for all electrostatics calculations. Heptamer formation of VchTnsC and EcoTnsC may be favoured by more efficient protomer-protomer packing, which appears less apparent in the hexameric ShoTnsC complex. f, Structural superposition of DNA-bound VchTnsC•ATP (this study) and EcoTnsC (PDB ID: 7MCS)22 monomers, by alignment of NTD residues 16–196 (VchTnsC) and residues 70–90 and 128–295 (EcoTnsC), yields an RMSD of 2.42 Å. The conformation of α1 (labelled) may create a more open protomer architecture enabling efficient packing of adjacent subunits, and thus, heptamer formation. ATP and AMPPNP are shown as sticks. g, Structural superposition of DNA-bound VchTnsC•ATP (this study) and ShoTnsC (PDB ID: 7M99)23 monomers, by alignment of NTD residues 16–196 (VchTnsC) and 34–198 (ShoTnsC), yields an RMSD of 1.27 Å. In ShoTnsC, the conformation of α1 may cause a more closed protomer architecture that obstructs efficient packing of adjacent subunits and promotes hexamer formation. ATP and ATPɣS are shown as sticks. h, Structural superposition of VchTniQ (PDB ID: 6V9P)46 and ShoTniQ monomers (PDB ID: 7N6I)23, which align with a global RMSD of 1.01 Å. ZnF motifs are indicated. i, Model of VchTniQ•TnsC interaction and its positioning relative to dsDNA, based on structural superpositions with the TniQ-bound TnsC complex from S. hofmannii (PDB ID: 7N6I; Methods)23, as shown in Fig. 5f. Protomers 3 and 4 of VchTnsC are shown in yellow and orange, respectively. Loops protruding from the N-terminal face of VchTnsC, which we found were essential for RNA-guided transposition (Extended Data Fig. 7b), are near the putative VchTniQ contact point. E69-D75 and A106–A113 are coloured dark orange and pink, respectively. j, An electrostatic rendering of VchTniQ-2 from the model shown in i suggests that DNA exiting the R-loop (dark grey) is well positioned to interact with a positively charged (blue) surface (left); TniQ-1 is shown in ribbon representation. DNA strand re-annealing may occur on this TniQ surface that is rich in lysine and arginine residues (right), guiding DNA from Cascade into the central pore of the VchTnsC heptamer. The 5′-CC-3′ PAM nucleotides are indicated by yellow dots.
