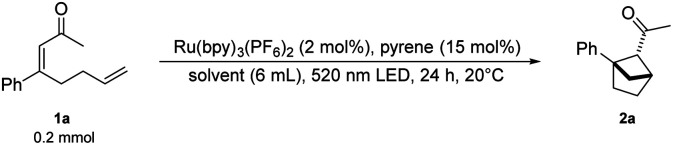
Optimization of the reaction condition.
| ||||
|---|---|---|---|---|
| Entrya | Sensitizer | Annihilator | Solvent | NMR yield |
| 1b | [Ru]2+ | Pyrene | SDS (4 wt%) | 62 |
| 2b | [Ru]2+ | Pyrene | Triton-X-100 (4 wt%) | 48 |
| 3b | [Ru]2+ | Pyrene | CTAC (4 wt%) | 12 |
| 4c | [Ru]2+ | Pyrene | SDS (4 wt%) | 76 (80d) |
| 5c | [Ru]2+ | Pyrene | Acetonitrile, no O2 | 52 |
| 6c | [Ru]2+ | Pyrene | DCM, no O2 | 45 |
| 7c | [Ru]2+ | Pyrene | MeOH, no O2 | 84 (79d) |
| 8c | [Ru]2+ | Pyrene | MeOH | 64 |
| 9c | [Ru]2+ | Pyrene | SDS (4 wt%), no O2 | 79 |
| 10c | [Ru]2+ | Pyrene | H2O, no O2 | 22 |
| 11c | [Ru]2+ | — | SDS (4 wt%) | 28 |
| 12c | — | Pyrene | SDS (4 wt%) | 0 |
| 13c | — | — | SDS (4 wt%) | 0 |
| 14e | [Ru]2+ | Pyrene | SDS (4 wt%) | 0 |
For the determination of the NMR yield, toluene (5.3 μL, 0.05 mmol) was used as internal standard.
3 W 520 nm LED.
10 W 520 nm LED.
Yields of isolated product.
No light. SDS = sodium dodecyl sulfate, CTAC = cetyltrimethylammonium chloride.
