Abstract
Background:
Prenatal exposure to endocrine-disrupting chemicals (EDCs) may disrupt normal fetal and postnatal growth. Studies have mainly focused on individual aspects of growth at specific time points using single chemical exposure models. However, humans are exposed to multiple EDCs simultaneously, and growth is a dynamic process.
Objective:
The objective of this study was to evaluate the associations between prenatal exposure to EDCs and children’s body mass index (BMI) growth trajectories using single exposure and mixture modeling approaches.
Methods:
Using data from the INfancia y Medio Ambiente (INMA) Spanish birth cohort (), prenatal exposure to persistent chemicals [hexachlorobenzene (HCB), 4-4′-dichlorodiphenyldichloroethylene (DDE), polychlorinated biphenyls (PCB-138, -150, and -180), 4 perfluoroalkyl substances (PFAS)] and nonpersistent chemicals (8 phthalate metabolites, 7 phenols) was assessed using blood and spot urine concentrations. BMI growth trajectories were calculated from birth to 9 years of age using latent class growth analysis. Multinomial regression was used to assess associations for single exposures, and Bayesian weighted quantile sum (BWQS) regression was used to evaluate the EDC mixture’s association with child growth trajectories.
Results:
In single exposure models exposure to HCB, DDE, PCBs, and perfluorononanoic acid (PFNA) were associated with increased risk of belonging to a trajectory of lower birth size followed by accelerated BMI gain by 19%–32%, compared with a trajectory of average birth size and subsequent slower BMI gain [e.g., relative risk ratio (RRR) per doubling in DDE (95% CI: 1.05, 1.35); RRR for (95% CI: 1.05, 1.66)]. HCB and DDE exposure were also associated with higher probability of belonging to a trajectory of higher birth size and accelerated BMI gain. Results from the BWQS regression showed the mixture was positively associated with increased odds of belonging to a BMI trajectory of lower birth size and accelerated BMI gain (odds ratio per 1-quantile increase of the ; credible interval: 1.03, 2.61), with HCB, DDE, and PCBs contributing the most.
Discussion:
This study provides evidence that prenatal EDC exposure, particularly persistent EDCs, may lead to BMI trajectories in childhood characterized by accelerated BMI gain. Given that accelerated growth is linked to a higher disease risk in later life, continued research is important. https://doi.org/10.1289/EHP11103
Introduction
Infant and early childhood growth and adiposity status are important factors in promoting healthy childhood development and well-being during adulthood.1 Children with overweight or obesity are more likely to continue in trajectories of excess weight during adulthood,2 putting them at an increased risk for complications, including diabetes, cardiovascular disease, and increased mortality.1–3
One risk factor of concern is exposure to endocrine-disrupting chemicals (EDCs), given that they may predispose individuals to develop obesity by interfering with normal endocrine function.4,5 Several EDCs are persistent and remain in the environment for many years through bioaccumulation [e.g., some organochlorine compounds (OCs), such as dichlorodiphenyldichloroethylene (DDE), hexaclorobenzene (HCB), polychlorinated bisphenyls (PCBs), and perfluoroalkyl substances (PFAS)].6 Others are nonpersistent (e.g., phthalates and phenols) and are metabolized quickly, but human exposure is constant owing to their widespread use.7,8 Exposure to EDCs occurs through the use of plastic convenience items, consumption of food and water, and application of personal care products, to name a few means.9,10 Exposure is particularly concerning for pregnant women because many EDCs have been shown to transfer in utero during a time period in which the fetus is particularly sensitive to alterations in their normal hormone environment.11,12 Numerous EDCs, both persistent and nonpersistent, may interfere with estrogenic, androgenic, glucocorticoid, and insulin signaling pathways,13 leading to impacts on normal fetal and postnatal growth14 and potentially resulting in increased disease susceptibility in later life.11,12,15
A number of studies have evaluated the impact of prenatal EDC exposure on birth size and later body mass index (BMI) in childhood, with most of these focusing on just one or two groups of EDCs,16–18 and only few including multiple EDCs from a wide range of chemical families.19–22 Fewer studies have used growth trajectories that integrate repeated measurements of fetal growth, birth size, or postnatal growth over time.23–27 By integrating multiple measures of growth from early life onward, the more dynamic aspects of growth can be captured, particularly during the first 2 y of life, which is a period of exponential growth.28 Capturing both prenatal (via birth size) and postnatal growth is important given that babies with high birth weight () and babies with low birth weight followed by accelerated growth are at an increased risk for obesity during childhood,29 which can negatively impact cardiometabolic risk factors.30 Studies that modeled growth trajectories found that prenatal exposure to certain phthalates was associated with the highest predicted BMI trajectories in a sex-specific manner from birth to 14 years of age,23 whereas prenatal DDE exposure was associated with increased growth from birth to 2 years of age.25 Most recently, a prospective birth cohort study of 1,118 mother–child pairs from Sweden found that prenatal exposure to a mixture of 41 EDC metabolites was associated with lower birth size and subsequent delayed growth from birth to 5 years of age.24 Given that children who experience accelerated growth have increased risks for chronic diseases in adulthood, such as hypertension, cardiovascular diseases, diabetes, and cancer,2,31–34 determining these childhood growth patterns and their determinants may enable intervention during early life, thus lessening future disease burden.
Studies evaluating the effects of prenatal EDC exposure on child growth have mainly focused on individual aspects of growth at specific time points (e.g., birth size, BMI) using single chemical exposure models. In real life, humans are exposed to multiple EDCs simultaneously and growth is a dynamic process; however, to our knowledge only one study24 has combined the use of a chemical mixture with multiple growth measures (i.e., growth trajectories). Therefore, the present study evaluated the associations between prenatal exposure to persistent and nonpersistent EDCs and children’s BMI growth trajectories from birth to 9 years of age through single exposure and mixture modeling approaches.
Methods
Study Population
This study included 1,911 mother–child pairs from the INfancia y Medio Ambiente (INMA) Spanish birth cohort study in Gipuzkoa (), Sabadell (), and Valencia (). Pregnant women were recruited during the first trimester through regional public hospitals between 2003 and 2008. The inclusion criteria included being years of age, a singleton pregnancy, intention to deliver at a reference hospital, and no assisted conception or communication issues.35 Mother–child pairs were followed-up during the third trimester, at birth, and at child ages 6 months and 1, 2, 4, 7, and 9 y. Information was collected through questionnaires and clinical examinations, including blood and urine samples35 Blood collected during the first trimester was centrifuged to separate serum, which was aliquoted into glass criotubes that were stored at . Urine was collected in polypropylene containers and aliquoted into polyethylene tubes and stored at . This study was approved by the ethics review boards of the hospitals involved, and mothers signed written consent for their and their child’s participation.
Measurement of Chemicals
Concentrations of 24 metabolites were determined using maternal serum, plasma, and urine samples taken during pregnancy. Concentrations of organochlorines (OCs: 4,4′-DDE, HCB, and PCB-138, -150, and -180) were measured in first trimester maternal serum samples. Samples were analyzed using gas chromatography with electron capture detection [GC-ECD; 5890 series II gas chromatograph equipped with split-splitless injector, ECD detector, and a 7673 autosampler (Agilent Technologies)] at the Gipuzkoa Basque Government Public Health Laboratory for Sabadell and Gipuzkoa and the Institute of Environmental Assessment and Water Research (IDAEA)/CSIC for Valencia, as described previously.36,37 Because OCs are lipophilic, concentrations were adjusted for maternal total serum lipid content, which was calculated from total cholesterol and triglycerides measured through enzymatic techniques.38
PFAS concentrations [perfluorohexanesulfonic acid (PFHxS), perfluorooctanoic acid (PFOA), perfluorooctanesulfonic acid (PFOS), and perfluorononanoic acid (PFNA)] were analyzed in first trimester maternal plasma samples. Samples were analyzed at the Institute for Occupational Medicine, Rheinisch-Westfälische Technische Hochschule (RWTH) Aachen University using column-switching high-performance liquid chromatography (HPLC) (Agilent 1100 Series HPLC apparatus with an additional isocratic Agilent G 1310A pump) coupled to tandem mass spectrometry (MS-MS; Sciex API 3000 LC-MS/MS system in electrospray ionization-negative mode). This information has been previously described.39
Phthalate metabolites [mono-ethyl phthalate (MEP), mono-iso-butyl phthalate (MiBP), mono--butyl phthalate (MnBP), monobenzyl phthalate (MBzP), mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), and mono-2-ethyl-5-carboxypentyl phthalate (MECPP)] were measured at the Norwegian Institute of Public Health (NIPH) for Gipuzkoa and Valencia using pooled urine samples from the first and third trimesters (pooled in equal volumes) with UHPLC-MS/MS (Triple Quad LC-MS/MS 6460 Series from Agilent Technologies),40 and at the Hospital de Mar Medical Research Institute (IMIM) for Sabadell using spot urine samples taken separately during the first and third trimesters using ultraperformance LC-MS/MS (Waters Corp.).41 Phenols, including four parabens [methyl paraben (MEPA), ethyl paraben (ETPA), propyl paraben (PRPA), and -butyl paraben (BUPA)], bisphenol A (BPA), benzophenone-3 (BP-3), and triclosan (TRCS) were measured at the Biosanitary Research Institute ibs.GRANADA for Gipuzkoa using pooled urine samples (first and third trimesters) using dispersive liquid–liquid microextraction followed by UHPLC-MS/MS,42 and at NIPH for Sabadell and Valencia using spot urine samples taken separately during the first and third trimesters with on-line solid-phase extraction followed by (UHPLC-MS/MS; Triple Quad MS/MS 6490; Agilent Technologies).43 Phthalate metabolites and phenols were adjusted for variation in urinary dilution by creatinine. In all cohorts, creatinine urine concentrations (derived from separate first and third trimester samples and averaged for analyses) were determined using the Jaffé method (kinetic with target measurement, compensated method; where a fixed concentration is subtracted from each result, with the assumption that the noncreatinine chromogen interference is constant between the samples), using a Beckman Coulter reactive in AU5400 (IZASA).44 In cases where pregnant women had only one sample from either the first or the third trimester, that one measurement was used as the average.
Details regarding laboratories, and limit of detection (LOD) or quantification (LOQ) by cohort and sampling period can be found in Tables S1–S3. For all concentrations, a value equal to the LOD divided by 2 was set to samples with concentrations below the LOD, and concentrations were transformed using the base-2 logarithm to achieve more normal distributions.
Growth Trajectories
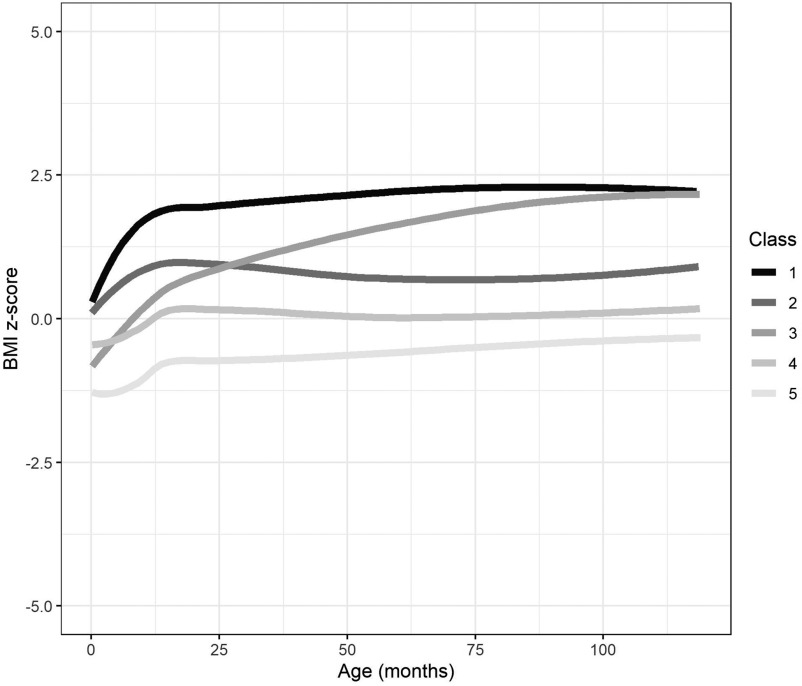
The growth trajectories used were calculated and already described in previously published work.45,46 Briefly, repeated measurements of each child’s height and weight were obtained from medical records and measurements taken by trained INMA personnel. Children were measured using standardized protocols, without shoes and in lightweight clothing. Age- and sex-specific BMI -scores were calculated using the World Health Organization Child Growth Standards.47,48 BMI -score trajectories (referred to as BMI trajectories hereafter) were estimated using latent class growth analysis (LCGA), specifically a latent class mixed model.45,49 Before estimating the trajectories, we checked whether the relationship between age and zBMI was linear (using the gamm function from the mgcv RStudio library to adjust a general additive mixed model). We found that with 3 degrees of freedom we had a good age function to modulate the relationship with zBMI. A possible of 2 to 7 trajectories were tested to find the best number of trajectories to represent the data. The method for selecting the best fit was based on Bayesian and Akaike information criteria and, additionally, that the trajectories found were meaningful (e.g., had frequency and that the trajectory displayed was distinct from the others). Ultimately, five distinct BMI trajectories were identified as the best fit for our data. Each trajectory included measurements taken from 0 to 9 years of age ( y), with an average of 14.3 measurement points per child. Detailed information on the RStudio package used and model parameters can be found in Table S4. The trajectories differed in birth size (defined as lower, average, or higher) and in BMI gain velocity (defined as slower or accelerated) (Figure 1; Table S11). Based on these definitions, the classes were labeled as follows: class 1, larger birth size with subsequent accelerated BMI gain; class 2, larger birth size with subsequent slower BMI gain; class 3, smaller birth size with subsequent accelerated BMI gain; class 4, average birth size with subsequent slower BMI gain; and class 5, smaller birth size with subsequent slower BMI gain. For analysis, class 4 was used as the reference category.
Figure 1.
BMI -score growth trajectories from 0 to 9 years of age () for the INMA birth cohort study. Class 1: higher birth size with accelerated BMI gain (, 11.1%). Class 2: higher birth size with slower BMI gain (, 26.8%). Class 3: lower birth size with accelerated BMI gain (, 15.3%). Class 4: average birth size with slower BMI gain (, 31.8%) (reference). Class 5: lower birth size with slower BMI gain (, 15.0%). See Table S11 for corresponding class-specific mean predicted trajectory values for each class by selected age groups. Note: BMI, body mass index; INMA, INfancia y Medio Ambiente.
Covariates
Information on covariates was obtained through interviewer-administered questionnaires answered by mothers at recruitment during the first trimester of pregnancy and included parity, smoking, socioeconomic status (SES), and maternal and paternal prepregnancy height and weight (which were used to calculate BMI). SES was derived from maternal and paternal self-reported occupation and recoded (using the higher of the two) using a widely used Spanish adaptation of the International ISCO88 coding system (, managers/technicians; , skilled; , semiskilled/unskilled).50 Maternal smoking during pregnancy was analyzed as “yes” if they indicated smoking during the first and/or third trimesters and “none” if they did not smoke. Maternal age at delivery (in years) was calculated using mother’s birth date and the child’s delivery date. Mediterranean diet score is an indicator of diet quality and was based on food frequency questionnaires taken during the first and third trimesters.51 Information on each child’s sex was obtained from clinical records.
Statistical Analysis
Descriptive statistics were used to summarize population averages. To handle missing values for the chemical exposures (not all participants included had all chemicals measured; missing ranged from 11.3% in the OCs to 48.7% in the phthalate metabolites) and covariates (missing ranged from 0.1% in maternal age to 5.9% in paternal BMI), we followed a multiple imputation approach under the assumption of the data being missing at random.52 Chemicals measured but found to be below the LOD were instead imputed as the LOD divided by 2, given that they were recorded as below LOD rather than as missing and to avoid generating an above-LOD value. We generated 20 imputed data sets using the ice command (with 10 cycles) in Stata. To improve prediction, variables included in the imputation procedure were all those to be included in the regression models [exposures (all 24 metabolites log-transformed), outcome (BMI trajectories), and covariates]. Covariates were selected for inclusion in the main analysis models based on previous knowledge45,53–58 and using a directed acyclic graph approach (Figure S1) and included parity (0 or ), SES, maternal and paternal prepregnancy BMI (in kilograms per meter squared), maternal age at delivery, maternal smoking during pregnancy, maternal Mediterranean diet score during pregnancy, child sex, and subcohort (Gipuzkoa, Sabadell, or Valencia). Further, additional predictor variables based on their relationship to the included covariates were included in imputation models59: maternal education (less than secondary or secondary completed), gestational weight gain (in kilograms), type of delivery (vaginal, cesarean, or instrumental), maternal country of birth (Spanish or foreigner), maternal previous breastfeeding (yes or no), child birth weight (in grams), child gestational age (in weeks), and child age at study follow-up (in years). For the imputation, continuous variables were treated using predictive mean matching, whereas binary and categorical variables were treated using logistic, ordinal, or multinomial models depending on variable type.
To estimate correlations between the chemical concentrations, Pearson correlation coefficients were calculated. Associations between chemical concentrations and BMI trajectories were analyzed by running single exposure models using multinomial logistic regression, which were exponentiated to relative risk ratios (RRRs) and using trajectory class 4 as the reference trajectory. Next, we tested the association between the chemical mixture, including concentrations and the BMI trajectories, using Bayesian weighted quantile sum (BWQS) regression. The BWQS regression is an extension of WQS regression that summarizes the overall exposure to the mixture by estimating a single weighted index while accounting for the individual contribution of each concentration of the mixture using weights.60 In addition, it allows for bidirectionality of the mixture coefficient, allowing more flexibility to the model and improving statistical power.60 Using the binomial function, each BMI trajectory was tested against the reference trajectory. We exponentiated the beta estimates to odds ratios (ORs) where the interpretation of the OR (X) is that for every 1-quartile increase in the BWQS weighted index there is an X increase in the odds of the tested trajectory compared with the reference trajectory. TRCS was not included in the mixture because it was not measured in the Gipuzkoa cohort. Given that BWQS regression gives each concentration a “weight” indicating their ranking in the mixture, we did not run individual analyses for each chemical subgroup.
As a sensitivity analysis, we repeated the single exposure models using complete case data. In addition, we conducted the following sensitivity analyses to better inform public health practices that may need to target specific vulnerable groups: a) stratification of single and multiple exposure models by child sex owing to the sex-specificity of some EDCs reported in the literature,16 and b) stratification of single and multiple exposure models by SES to evaluate vulnerability to EDC effects.53 Statistical significance was defined as a confidence interval (CI) level of 95%. Imputation and regression analyses were done using Stata (version 14). BWQS regression was conducted using RStudio (version 4.0.3).
Results
Study Population
Figure 1 and Table 1 show the distribution of BMI trajectories and the main characteristics for the study population ( mother–child pairs). The majority of children (32%) belonged to the reference BMI trajectory, class 4—average birth size and slower BMI gain, whereas the least number of children (11%) belonged to class 1—larger birth size and accelerated BMI gain (Figure 1). No differences were found in the main characteristics between unimputed and imputed data sets (Table S5). Population characteristics were generally consistent across the BMI trajectories with a few differences (Table 1). When compared with all BMI trajectories combined, class 1 had a higher percentage belonging to the low SES group and mothers who smoked during pregnancy. In addition, more mothers with children in class 5 were nulliparous, and maternal and paternal prepregnancy BMI were slightly higher in classes 1 and 3 (Table 1). The geometric means (GMs) of the EDC concentrations were generally similar between the unimputed and the imputed data sets (Table 2). Correlations between the chemical exposures were generally high within distinct classes of chemicals, namely phthalate metabolites, PFAS, and PCBs, but not between one another (Figures S2–S3).
Table 1.
Characteristics [ (%) or ] of the study population () for the INMA birth cohort study.
| Characteristic | Missing | Unimputed data | Class 1: higher birth size with accelerated BMI gain | Class 2: higher birth size with slower BMI gain | Class 3: lower birth size with accelerated BMI gain | Class 4: average birth size with slower BMI gain | Class 5: lower birth size with slower BMI gain |
|---|---|---|---|---|---|---|---|
| (11.1%) | (26.8%) | (15.3%) | (31.8%) | (15.0%) | |||
| Subcohort | 0 | — | — | — | — | — | — |
| Gipuzkoa | — | 556 (29.1) | 59 (27.8) | 168 (32.8) | 83 (28.4) | 173 (28.5) | 73 (25.4) |
| Sabadell | — | 659 (34.5) | 68 (32.1) | 170 (33.1) | 88 (30.1) | 226 (37.2) | 107 (37.3) |
| Valencia | — | 696 (36.4) | 85 (40.1) | 175 (34.1) | 121 (41.44) | 208 (34.3) | 107 (37.3) |
| SES | 94 | — | — | — | — | — | — |
| High | — | 594 (32.7) | 47 (23.5) | 154 (31.8) | 97 (34.6) | 202 (34.8) | 94 (34.6) |
| Middle | — | 477 (26.2) | 57 (28.5) | 129 (26.6) | 66 (23.6) | 157 (27.1) | 68 (25.0) |
| Low | — | 746 (41.1) | 96 (48) | 202 (41.7) | 117 (41.8) | 221 (38.1) | 110 (40.4) |
| Smoking during pregnancy | 33 | — | — | — | — | — | — |
| None | — | 1,293 (68.9) | 125 (59.8) | 360 (71.0) | 194 (67.4) | 417 (69.7) | 197 (71.4) |
| Yes | — | 585 (31.1) | 84 (40.2) | 147 (29.0) | 94 (32.6) | 181 (30.3) | 79 (28.6) |
| Parity | 96 | — | — | — | — | — | — |
| 0 | — | 1,012 (55.8) | 109 (54.5) | 234 (48.4) | 173 (61.8) | 316 (54.5) | 180 (66.4) |
| — | 803 (44.2) | 91 (45.5) | 250 (51.7) | 107 (38.2) | 264 (45.5) | 91 (33.6) | |
| Maternal age at delivery (y) | 2 | ||||||
| Maternal prepregnancy BMI () | 17 | ||||||
| Paternal BMI (prepregnancy) () | 112 | ||||||
| Maternal Mediterranean diet score | 94 | ||||||
| Child sex | 0 | — | — | — | — | — | — |
| Female | — | 927 (48.5) | 85 (40.1) | 242 (47.2) | 143 (49.0) | 326 (53.7) | 131 (45.6) |
| Male | — | 984 (51.5) | 127 (59.9) | 271 (52.8) | 149 (51.0) | 281 (46.3) | 156 (54.4) |
Note: —, not applicable; BMI, body mass index; INMA, INfancia y Medio Ambiente; N, number of observations; SD, standard deviation; SES, socioeconomic status.
Table 2.
Summary statistics for the EDCs in the unimputed and imputed data sets for the INMA birth cohort study.
| EDC | Unimputed data | Imputed data () | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Complete name | Abbreviation | Percentage missing (%) | Percentage (%) | P25 | P50 | P75 | Max | GM (95% CI) | GM (95% CI) | |
| Phthalates | ||||||||||
| Mono-ethyl phthalate | MEP | 983 | 48.6 | 0.1 | 109.94 | 239.63 | 522.5 | 10,952 | 243.33 (226.88, 260.97) | 249.90 (247.15, 252.68) |
| Mono-iso-butyl phthalate | MiBP | 983 | 48.6 | 0 | 20.28 | 29.6 | 44.38 | 1,614 | 31.81 (30.45, 33.23) | 34.43 (34.19, 34.67) |
| Mono--butyl phthalate | MnBP | 983 | 48.6 | 0.5 | 17.29 | 26.84 | 46.66 | 3,597 | 31.49 (29.53, 33.58) | 33.64 (33.30, 33.98) |
| Monobenzyl phthalate | MBzP | 982 | 48.6 | 0.6 | 5.21 | 9.37 | 16.12 | 405.1 | 9.45 (8.95, 9.99) | 10.47 (10.38, 10.56) |
| Mono-2-ethylhexyl phthalate | MEHP | 981 | 48.7 | 0.4 | 5.04 | 8.38 | 13.94 | 403.7 | 8.43 (8.00, 8.88) | 10.31 (10.23, 10.39) |
| Mono-2-ethyl-5-hydroxyhexyl phthalate | MEHHP | 983 | 48.6 | 0 | 15.25 | 24.43 | 40.04 | 1,060 | 25.24 (24.07, 26.47) | 32.21 (31.98, 32.44) |
| Mono-2-ethyl-5-oxohexyl phthalate | MEOHP | 983 | 48.6 | 0 | 10.51 | 16.54 | 26.68 | 756 | 17.23 (16.44, 18.05) | 21.77 (21.62, 21.92) |
| Mono-2-ethyl-5-carboxypentyl phthalate | MECPP | 983 | 48.6 | 0.1 | 24.15 | 36.56 | 53.42 | 1,486 | 38.22 (36.67, 39.84) | 46.83 (46.54, 47.13) |
| Phenols | ||||||||||
| Methyl paraben | MEPA | 1,148 | 39.9 | 0 | 67.81 | 204.63 | 461 | 45,927 | 185.06 (168.53, 203.20) | 180.98 (178.12, 183.87) |
| Ethyl paraben | ETPA | 1,148 | 39.9 | 0.5 | 4.14 | 13.78 | 43.13 | 3,754 | 13.17 (11.90, 14.58) | 13.05 (12.82, 13.27) |
| Propyl paraben | PRPA | 1,146 | 40.0 | 0.8 | 12.47 | 40.96 | 117.6 | 14,132 | 36.08 (32.43, 40.14) | 34.49 (33.87, 35.12) |
| -butyl paraben | BUPA | 1,146 | 40.0 | 13.1 | 0.49 | 2.67 | 9.59 | 428.3 | 2.09 (1.86, 2.35) | 2.05 (2.01, 2.09) |
| Bisphenol A | BPA | 1,147 | 40.0 | 5.1 | 1.87 | 3.18 | 5.99 | 594.1 | 2.98 (2.74, 3.24) | 2.87 (2.83, 2.91) |
| Benzophenone-3 | BP-3 | 1,148 | 39.9 | 0.7 | 1.32 | 4.48 | 24.48 | 10,029 | 6.51 (5.76, 7.35) | 6.45 (6.32, 6.58) |
| Triclosana | TRCS | 893 | 34.1 | 0 | 6.21 | 34.44 | 130.6 | 1,958 | 30.04 (26.53, 34.01) | 29.70 (29.07, 30.35) |
| Organochlorine compounds | ||||||||||
| Hexachlorobenzene | HCB | 1,695 | 11.3 | 7.7 | 24.07 | 44.93 | 76.73 | 2,130 | 41.02 (39.21, 42.91) | 40.95 (40.58, 41.34) |
| 4,4′-Dichlorodiphenyl dichloroethylene | DDE | 1,695 | 11.3 | 0.9 | 75.91 | 123.15 | 212.2 | 21,141 | 132.69 (127.08, 138.54) | 134.64 (133.45, 135.85) |
| 2,2′,3,4,4′,5′-Hexachlorobiphenyl | PCB-138 | 1,694 | 11.4 | 11.3 | 16.94 | 26.76 | 40 | 690.4 | 24.68 (23.80, 25.59) | 24.43 (24.25, 24.61) |
| 2,2′,4,4′,5,5′-Hexachlorobiphenyl | PCB-153 | 1,694 | 11.4 | 4.6 | 29.9 | 44.58 | 63.44 | 484 | 40.29 (38.85, 41.78) | 39.62 (39.32, 39.91) |
| 2,2′,3,4,4′,5,5′-Heptachlorobiphenyl | PCB-180 | 1,695 | 11.3 | 7.6 | 20.37 | 31.89 | 47.8 | 258.2 | 28.89 (27.84, 29.97) | 28.40 (28.19, 28.62) |
| Perfluoroalkyl substances | ||||||||||
| Perfluorohexanesulfonic acid | PFHxS | 1,236 | 35.3 | 3.7 | 0.41 | 0.58 | 0.82 | 11 | 0.57 (0.55, 0.58) | 0.55 (0.55, 0.55) |
| Perfluorooctanoic acid | PFOA | 1,236 | 35.3 | 0 | 1.63 | 2.35 | 3.3 | 31.6 | 2.31 (2.24, 2.38) | 2.26 (2.24, 2.27) |
| Perfluorooctanesulfonic acid | PFOS | 1,236 | 35.3 | 0 | 4.52 | 6.06 | 7.82 | 38.6 | 5.78 (5.63, 5.93) | 5.69 (5.66, 5.72) |
| Perfluorononanoic acid | PFNA | 1,236 | 35.3 | 0.7 | 0.49 | 0.66 | 0.9 | 5.5 | 0.65 (0.63, 0.67) | 0.64 (0.64, 0.64) |
Note: Phthalates and phenols are in micrograms per gram creatinine. Organochlorine compounds are in nanograms per gram lipid. Perfluoroalkyl substances are in nanograms per milliliter. LOD/LOQ values are located in Tables S1–S3. BMI, body mass index; CI, confidence interval; EDC, endocrine-disrupting chemical; GM, geometric mean; INMA, INfancia y Medio Ambiente; LOD, limit of detection; LOQ, limit of quantification; Max, maximum; N, number of observations; P, percentile.
TRCS measurement was only available for Sabadell and Valencia, .
Single Exposure Models
Associations from single exposure models and BMI trajectories, in reference to class 4 (average birth size followed by slower BMI gain), are shown in Figure 2 and Table S6. Prenatal exposure to all individual OCs (HCB, DDE, and PCB-138, -153, and -180) was associated with a statistically significant increase in risk of belonging to the BMI trajectory of lower birth size followed by accelerated BMI gain (class 3) by 19%–25% per doubling of exposure concentration [e.g., class 3 vs. 4: DDE (95% CI: 1.05, 1.35); HCB (95% CI: 1.09, 1.42)]. In addition, HCB and DDE concentrations were associated with an increased risk of belonging to the BMI trajectory of higher birth size followed by accelerated BMI gain (class 1) [class 1 vs. 4: HCB (95% CI: 1.01, 1.36); DDE (95% CI: 1.00, 1.32)]. Regarding PFAS, a doubling of prenatal PFNA exposure was associated with a 32% increase in risk of belonging to class 3 (class 3 vs. 4: ; 95% CI: 1.05, 1.66); for other PFAS, RRR estimates for this class were increased but did not reach statistical significance. No significant associations were observed for any phthalate metabolites or phenols.
Figure 2.
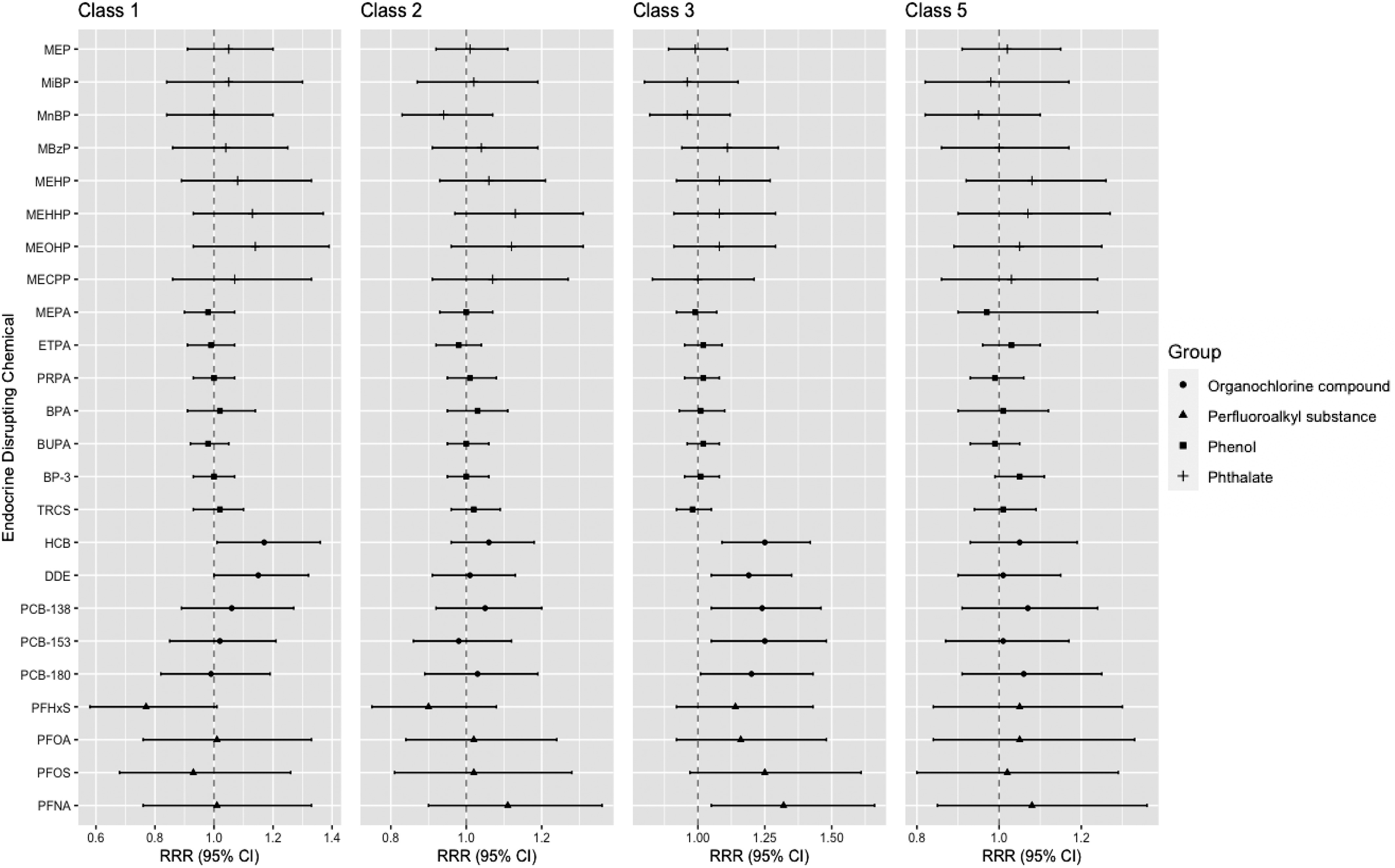
Associations (RRRs and 95% CIs) between prenatal EDC exposure and BMI trajectories: single exposure models () for the INMA birth cohort study. BMI trajectory descriptions: class 1, higher birth size with accelerated BMI gain; class 2, higher birth size with slower BMI gain; class 3, lower birth size with accelerated BMI gain; class 4, average birth size with slower BMI gain (reference); class 5, lower birth size with slower BMI gain. For EDC complete names, see Table 2. All RRRs are in reference to class 4. Associations were determined using imputed data (). RRRs are from multinomial logistic regression models adjusted for child sex, parity, SES, maternal and paternal BMI, maternal smoking, maternal age, Mediterranean diet score, and subcohort. See Table S6 for corresponding numeric data. Note: BMI, body mass index; CI, confidence interval; EDC, endocrine-disrupting chemical; INMA, INfancia y Medio Ambiente; RRR, relative risk ratio.
In complete case analyses, results from the single exposure models were similar or somewhat stronger than imputed models and had the same directionality (Table S7). In addition to the statistically significant findings of the imputed models, the complete case analyses showed statistically significant associations between MBzP, MEHP, PFOA, and PFOS concentrations and increased probability of belonging to class 3.
In stratified analyses by sex, associations generally followed a pattern similar to that of the overall population, with increases in risk of belonging to class 3 seen for OCs and PFAS in females and males, although these did not always reach statistical significance (Table S8). Statistically significantly increased RRRs for class 3 were observed in males for HCB, DDE, and PCB-153 [class 3 vs. 4: (95% CI: 1.06, 1.52), 1.30 (95% CI: 1.10, 1.54), and 1.26 (95% CI: 1.00, 1.59), respectively], and in females for HCB (; 95% CI: 1.01, 1.49). In males, exposure to DDE was associated with increased risk of belonging to class 1 vs. 4: (; 95% CI: 1.01, 1.46). Although not significant in overall analyses, MEHHP exposure was associated with an increased probability of belonging to the trajectory of higher birth size followed by slower BMI gain (class 2) in females [class 2 vs. 4: (; 95% CI: 1.01, 1.51)].
In stratified analyses by SES, associations varied depending on the EDC and no one particular SES group was consistently at a higher risk than others (Table S9). Statistically significant risk increases of belonging to classes 1 and 3 were seen for HCB in those of middle SES [ (95% CI: 1.08, 2.15) and 1.53 (95% CI: 1.13, 2.07), respectively]. Although increased RRRs were found for DDE and class 1 in children of low SES (; 95% CI: 1.03, 1.48) and class 3 in children of high SES (; 95% CI: 1.07, 1.80). Associations for PCBs were generally higher among children of high SES, with PCB-138 and -153 and class 3 reaching statistical significance [ (95% CI: 1.02, 2.14) and 1.48 (95% CI: 1.02, 2.14), respectively]. PFAS exposures were associated with higher RRRs in both low and high SES groups, particularly within classes 1 and 3, and association of PFNA exposure and belonging to class 3 reached statistical significance among those of low SES (; 95% CI: 1.07, 2.07). Although not significant in overall analyses, in children of low SES exposure to MEHP, MEHHP, and MEOHP was associated with increased probability of belonging to a BMI trajectory of lower birth size followed by slower BMI gain (class 5), and class 2 for MEHHP and MEOHP only [e.g., class 5 vs. 4: MEHP (95% CI: 1.01, 1.67); class 2 vs. 4: MEOHP (95% CI: 1.03, 1.81)].
Multiple Exposure Models
In mixture models using BWQS regression, the mixture effect was positively associated with increased odds of belonging to BMI trajectory class 3 in reference to class 4 [OR per 1-quantile increase of the EDC ; 95% credible interval (CrI): 1.03, 2.61] (Table 3). Components of HCB; DDE; PCB-138, -153, -180; and BP-3 had somewhat higher weights (in that order) and thus contributed slightly more to the overall mixture (Table S10). No associations between the EDC mixture and the other BMI trajectory classes were observed (Table 3). When stratifying by sex, the EDC mixture was positively associated with BMI trajectory class 2 in females (; 95% CrI: 1.03, 2.41) but not males (; 95% CrI: 0.50, 1.25) (Table 3). In stratification by SES, the EDC mixture was associated with the class 3 BMI trajectory in children of low SES (; 95% CrI: 1.28, 4.44), whereas there was no evidence for an association in children from middle or high SES groups ( and 1.05, respectively).
Table 3.
Associations between exposure to prenatal EDC mixture and BMI trajectories from BWQS regression expressed as ORs (95% CrIs) using imputed data (), given unstratified and stratified by sex and socioeconomic status; for the INMA birth cohort study.
| Models | Class 1: higher birth size with accelerated BMI gain | Class 2: higher birth size with slower BMI gain | Class 3: lower birth size with accelerated BMI gain | Class 4: average birth size with slower BMI gain | Class 5: lower birth size with slower BMI gain |
|---|---|---|---|---|---|
| Unstratifieda | 1.15 (0.68, 1.97) | 1.16 (0.86, 1.58) | 1.70 (1.03, 2.61) | Ref | 1.23 (0.83, 1.88) |
| Sexb | |||||
| Female | 1.38 (0.61, 3.10) | 1.57 (1.03, 2.41) | 1.43 (0.81, 2.59) | Ref | 1.32 (0.76, 2.32) |
| Male | 1.11 (0.61, 2.08) | 0.79 (0.50, 1.25) | 1.84 (0.88, 3.71) | Ref | 1.08 (0.63, 1.92) |
| SESc | |||||
| High | 1.68 (0.66, 4.31) | 1.02 (0.63, 1.72) | 1.05 (0.49, 1.44) | Ref | 1.23 (0.63, 2.44) |
| Middle | 0.68 (0.26, 1.97) | 1.39 (0.61, 2.23) | 1.09 (0.47, 2.72) | Ref | 0.90 (0.38, 2.08) |
| Low | 1.25 (0.67, 2.41) | 1.38 (0.86, 2.27) | 2.32 (1.28, 4.44) | Ref | 1.42 (0.73, 2.72) |
Note: Using data from the first imputed data set. BMI, body mass index; BWQS, Bayesian weighted quantile sum; CrI, credible interval; EDC, endocrine-disrupting chemical; INMA, INfancia y Medio Ambiente; N, number of observations; OR, odds ratio; Ref, reference; SES, socioeconomic status.
Unstratified models adjusted for child sex, parity, SES, maternal and paternal BMI, maternal smoking, maternal age, Mediterranean diet score, and subcohort.
Sex-stratified models adjusted for the same as unstratified models with the exception of sex.
SES-stratified models adjusted for the same as unstratified models with the exception of SES.
Discussion
In this large prospective cohort of mother–child pairs in Spain, HCB, DDE, PCBs, and PFNA were associated with an increased risk of belonging to a BMI trajectory of lower birth size and subsequent accelerated BMI gain. This association was also observed when considering the whole mixture of 23 EDCs, with OCs contributing the most to the mixture effect. The EDC mixture effect was strongest and statistically significant in those of low SES. Further, HCB and DDE exposure was associated with higher probability to belong to a BMI trajectory of higher birth size and accelerated BMI gain.
Our findings suggest that prenatal exposure to HCB and DDE was associated with the two trajectories characterized by accelerated BMI gain (starting from lower and higher birth size), and that PCB exposure was associated with the trajectory of lower birth size and subsequent accelerated BMI gain. These findings are largely in line with existing literature. A pooled analysis on four Flemish birth cohorts found that prenatal DDE exposure was associated with increased birth weight,19 although we too found DDE (and HCB) exposure to be associated with a trajectory characterized by higher birth size, we also found those exposures linked to a different trajectory characterized by lower birth size. Given that both of the trajectories are followed by subsequent accelerated BMI gain, this likely indicates that these exposures are more likely to be associated by accelerated BMI gain in children independent of birth size. In the same Flemish study, an inverse association was found for birth weight and congeners of PCBs19 (a finding previously described in a meta-analysis of 12 European birth cohorts61). Further, an earlier study using INMA data found that prenatal exposure to HCB and DDE was associated with rapid growth during the first 6 months of life; however, associations were null for prenatal exposure to PCBs.62 A pooled study from seven European cohorts found that prenatal DDE exposure was associated with increased growth rate (change in weight-for-age -score) from birth to 2 years of age.25 A review highlighted that exposure to HCB and DDE was consistently associated with increased BMI during childhood, but that evidence is less clear for PCBs.17 These previous works studied birth size and childhood weight/growth parameters independently. Our study combined these growth parameters in the same trajectory and provides novel evidence to support a relationship between prenatal exposure to HCB, DDE, and PCBs and accelerated BMI gain.
Regarding PFAS, to our knowledge our study is the first to use BMI trajectories beginning from birth and find that exposure to PFAS, especially PFNA, was associated with a higher probability of belonging to a trajectory of lower birth size and accelerated BMI gain. A recent review and meta-analysis on persistent EDCs included only legacy PFAS (PFOA and PFOS) and did not find conclusive evidence for an association between these chemicals and childhood obesity.17 However, previous reviews did conclude that PFAS were associated with a rapid increase in BMI during childhood,63 as well as with excess adiposity and risk of obesity during childhood.15,64 Further, a review highlighted that PFAS levels during pregnancy were associated with decreases in birth weight.65 This relationship may be due to reverse causation bias in studies where PFAS were analyzed in blood samples taken during late pregnancy.66 However, our study used samples taken during the first trimester, so this bias is unlikely to have affected our results.
Regarding the nonpersistent EDCs—phthalates and phenols (including parabens)—reviews on the topic have indicated largely mixed results with regard to prenatal exposure and later BMI.15,64 A recent systematic review and meta-analysis found a significant negative relationship between di(2-ethylhexyl) phthalate and later BMI -score while also reporting inconsistent results between phthalates overall and later BMI in their review.67 In terms of growth measures outside of BMI, the INMA study previously reported a negative association with exposure to high-molecular-weight phthalates and rapid growth during infancy in males,41 whereas other studies reported positive associations with rapid growth during infancy and higher probability of following an overweight trajectory from infancy to adolescence in a sex-specific and dose-specific manner.23,26,27 We did not observe any results with any of the phenols. BPA is the most frequently studied phenol, but results are inconsistent.15,64
The only previous study that investigated an EDCs mixture (which included phthalates, plasticizers, bisphenols, TRCS, polycyclic aromatic hydrocarbons, pesticides, PFAS, OCs, and PCBs) with children’s growth parameters from their weight trajectory found a positive association with a mixture that included 41 metabolites and lower birth weight -scores and slower weight gain parameters until 5.5 years of age.24 They reported that TRCS and PFOA were primary drivers of these associations given that they had the highest WQS weights. Similar to our study, Svensson et al.24 found an association with lower birth size associated with their EDC mixture. However, contrary to our results, they found their mixture was associated with subsequent slower or delayed growth, whereas we found our mixture was associated with subsequent accelerated growth. This difference could be due several factors, such as the following:
Differences in study population: Although both studies come from Europe and the mothers had similar age and BMI, the SELMA study size was somewhat smaller ( vs. our study at 1,911). The SELMA study reported higher SES, with 64% reporting college education, whereas INMA has only 32.7% in the higher SES category (assuming education and SES are related to one another), and INMA reported more maternal smoking (31.1% vs. SELMA’s 5.5%). Although sample size may not have contributed much to the differences observed, SES and smoking are two factors known to affect chemical exposure.
Differences in metabolites included in the mixture: The SELMA study included 41 metabolites, whereas we included 23. Although chemicals were from similar chemical groups, one of the primary drivers in the SELMA study (TRCS, an antibacterial and antifungal agent) was not included in our mixture analysis because one the cohorts did not measure it. In single exposure models, where we did analyze TRCS, we did not observe significant results between TRCS and any of the growth trajectories.
Child age: The SELMA study used growth until 5.5 years of age, whereas we used growth until 9 years of age. Including growth for an additional 4 y may have allowed us to calculate more precise trajectories given that the children were older; however, in both studies, the children were prepubertal, so it is unlikely the differences observed are due to this.
Calculation of the growth parameters: We used LCGA to estimate BMI growth trajectories, whereas the SELMA study used a double-logistic growth model and extracted growth parameters of weight rather than BMI (infant growth spurt rate and peak growth velocity).
Mixture model selected: The SELMA study chose WQS regression, whereas we selected BWQS regression. Although similar, BWQS regression builds on the framework of WQS regression to increase model flexibility. Given that ours are the first studies to analyze EDC mixtures with growth trajectories, it is important for the findings to be replicated in other studies.
In addition, we stratified our results by sex and SES. In single exposure models we did not observe strong patterns between the stratified groups; however, in the mixture exposure models we observed a statistically significant association with the trajectory of lower birth size and accelerated BMI gain in those of low SES, whereas there was no evidence of an association in the middle or high SES groups. Previous work on the social determinants of EDC exposure has reported results that support higher exposure to OCs in higher SES groups.53,57,68 However, research has reported mixed results with PFAS and phthalates and SES.69–71 Given that our mixture was driven by OCs and research has shown OC concentrations (primarily PCBs) are higher in higher SES groups, we had expected to find significant results with the high SES group; however, we found the opposite. This may indicate that although higher SES groups may have higher levels of certain EDCs, individuals of lower SES groups may be more vulnerable to EDC effects due to other factors. Currently, research is lacking in this area; however, this is an important finding and future studies should focus on groups that may be more vulnerable, including those of low SES. Our analyses stratifying the mixture models by sex did not indicate large differences between males and females, even though results were somewhat higher in males for class 3 and in females for class 2. Differences in sex may be attributable to effects on sex hormones (e.g., estrogens and androgens) that play a role in adipogenesis.
The exact mechanism by which prenatal exposure to EDCs may affect childhood growth is unclear, and it may differ depending on the chemical in question. Nonetheless, the obesogen hypothesis states that EDCs interfere with the endocrine and metabolic systems, thus altering normal growth patterns, weight gain, and obesity.72 Supporting literature has identified the peroxisome proliferator-activated receptor (PPAR)-alpha and -gamma pathways as key contributors. These receptors regulate lipid metabolism, healthy placenta function, and fetal and child development, indicating their ability to influence child growth starting from prenatal life.73 In addition, EDCs have been found to be able to induce adipogenic differentiation and to decrease metabolic efficiency, both of which can lead to obesity.64,74,75
This study has some strengths worth mentioning. The data used is part of a large collaborative longitudinal study in Spain that began following mother–child pairs from the beginning of pregnancy. The multiple follow-up points throughout infancy and childhood up to 9 years of age enabled the calculation of BMI trajectories, another strength of this study. By using LCGA, we were able to assign BMI trajectory classes to our population, defined by specific growth parameters that could be used for later regression analyses. This method offers ease of interpretation, but it may assign a child to a BMI trajectory they do not really follow, although that would be the minority. We also included a mixture of EDCs that included phthalates, phenols, OCs, and PFAS. This wide variety of chemicals does not include all potentially EDCs. However, it offers a good representation of those that may affect childhood growth and those that humans are commonly exposed to. In addition, we were able to use a novel mixture approach, BWQS regression, which allowed us to analyze an EDC mixture more representative of real-life exposure and to identify those chemicals that were driving the associations (e.g., those with the highest weights).
Our study should be interpreted with the following limitations in mind. The nonpersistent chemicals in this study were analyzed using only one or two spot urine samples or a pooled urine sample. These types of chemicals have large variability in sample concentrations, which can lead to measurement error on the exposure variable and, ultimately, bias associations toward the null owing to regression dilution bias.76 This is not the case for the persistent EDCs in our study (i.e., OCs and PFAS), which have long half-lives and likely give a reliable estimate of exposure. Further, there were missing values for many exposures in our study. To correct for this, we used multiple imputation under the missing at random assumption, which has been proven to give valid results, rather than use complete case analysis, which can lead to selection bias.59 By imputing the exposures, we were also able to maintain a sufficient sample for BWQS regression. Multiple imputation should be a technique considered in future studies aiming to use mixture analysis, but it may have missing exposure values. However, a limitation to using imputed data was that although we stratified our results by sex and SES, we could not formally test for interaction in the multinomial logistic regression or BWQS models given that the current statistical software does not support testing with an imputed data set. Finally, multiple comparison issues are important in single exposure models. We did not adjust for multiple testing in our study because this can be overconservative (i.e., reduce type I error at the expense of not detecting associations). Instead, the single exposure models compliment the mixture models, reducing copollutant confounding by simultaneously taking into account the exposure variables. When looking at our results we found similar results between the significant EDCs in the single exposure models and those with the highest weights in BWQS regression, giving confidence to our results. We based our main conclusions on the similarities between these results.
Conclusions
Our results demonstrate that early life exposure to environmental factors may potentially influence a child’s growth trajectory. Our study found that chemical exposures and their mixture were related to an increased risk of belonging to a trajectory of lower birth size followed by accelerated BMI gain, and for some chemicals (HCB and DDE) by higher birth size and accelerated BMI gain. In both single and mixture EDC models, persistent EDCs seemed to be the EDCs of most concern, and populations of low SES appeared to be more vulnerable to the EDC mixture. Given that accelerated growth has been linked to adverse health consequences (e.g., hypertension, obesity, cardiovascular diseases, diabetes, cancers) in later life, it would be important for future research to evaluate the health impacts of prenatal EDC exposure over the life course.32
Supplementary Material
Acknowledgments
We thank all of the study participants for their support with the INfancia y Medio Ambiente (INMA) birth cohort studies. We also thank the dedicated INMA research nurses and staff for their work in conducting the study visits.
This study received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 874583, the ATHLETE (Advancing Tools for Human Early Lifecourse Exposome Research and Translation) project, and grant agreement no. 825712, the OBERON project (both to M.V.).
M.C. and M.G. hold Miguel Servet fellowships (MS16/00128, CPII18/00018, to M.C.) funded by Instituto de Salud Carlos III and co-funded by the European Social Fund “Investing in Your Future.”
Instituto de Salud Global de Barcelona (ISGlobal) acknowledges support from the Spanish Ministry of Science and Innovation and the State Research Agency through the “Centro de Excelencia Severo Ochoa 2019–2023” Program (CEX2018-000806-S) and support from the Generalitat de Catalunya through the CERCA Program.
INMA Gipuzkoa: This study was funded by grants from the Instituto de Salud Carlos III (FIS-PI06/0867, FIS-PI09/00090, FIS-PI13/02187, and FIS-PI18/01142, including FEDER funds), Spanish Consortium for Research on Epidemiology and Public Health (CIBERESP), Department of Health of the Basque Government (2005111093, 2009111069, 2013111089, and 2015111065), and the Provincial Government of Gipuzkoa (DFG06/002, DFG08/001, and DFG15/221), and annual agreements with the municipalities of the study area (Zumarraga, Urretxu, Legazpi, Azkoitia y Azpeitia y Beasain).
INMA Valencia: This study was funded by grants from the European Union (FP7-ENV-2011 cod 282957 and HEALTH.2010.2.4.5-1), Spain: ISCIII (Red INMA G03/176, CB06/02/0041; FIS-FEDER: PI03/1615, PI04/1509, PI04/1112, PI04/1931, PI05/1079, PI05/1052, PI06/1213, PI07/0314, PI09/02647, PI11/01007, PI11/02591, PI11/02038, PI13/1944, PI13/2032, PI14/00891, PI14/01687, PI16/1288, and PI17/00663; Miguel Servet-FEDER CP11/00178, CP15/00025, and CPII16/00051), Generalitat Valenciana: FISABIO (UGP 15-230, UGP-15-244, and UGP-15-249), and the Alicia Koplowitz Foundation 2017.
INMA Sabadell: This study was funded by grants from the Instituto de Salud Carlos III (Red INMA G03/176; CB06/02/0041; PI041436; PI081151, including FEDER funds; PI12/01890, including FEDER funds; CP13/00054, including FEDER funds; PI15/00118, including FEDER funds; CP16/00128, including FEDER funds; PI16/00118, including FEDER funds; PI16/00261, including FEDER funds; PI17/01340, including FEDER funds; PI18/00547, including FEDER funds), CIBERESP, Generalitat de Catalunya-CIRIT 1999SGR 00241 (to P.M.), Generalitat de Catalunya-AGAUR (2009 SGR 501 and 2014 SGR 822), Fundació La marató de TV3 (090430), Spanish Ministry of Economy and Competitiveness (SAF2012-32991, including FEDER funds), Agence Nationale de Securite Sanitaire de l’Alimentation de l’Environnement et du Travail (1262C0010; EST-2016 RF-21), EU Commission (261357, 308333, 603794, and 634453).
References
- 1.WHO (World Health Organization). 2021. Fact sheet: cardiovascular diseases (CVDs). https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) [accessed 20 July 2021].
- 2.Barker DJP, Osmond C, Forsén TJ, Kajantie E, Eriksson JG. 2005. Trajectories of growth among children who have coronary events as adults. N Engl J Med 353(17):1802–1809, PMID: , 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- 3.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, et al. . 2008. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 371(9609):340–357, PMID: , 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heindel JJ, Blumberg B. 2019. Environmental obesogens: mechanisms and controversies. Annu Rev Pharmacol Toxicol 59:89–106, PMID: , 10.1146/annurev-pharmtox-010818-021304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heindel JJ, Newbold R, Schug TT. 2015. Endocrine disruptors and obesity. Nat Rev Endocrinol 11(11):653–661, PMID: , 10.1038/nrendo.2015.163. [DOI] [PubMed] [Google Scholar]
- 6.Downie D, Templeton J. 2014. Persistent organic pollutants. In: Routledge Handbook of Global Environmental Politics. Chapter 35. New York, NY: Routledge. [Google Scholar]
- 7.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. . 2009. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 30(4):293–342, PMID: , 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wittassek M, Koch HM, Angerer J, Brüning T. 2011. Assessing exposure to phthalates—the human biomonitoring approach. Mol Nutr Food Res 55(1):7–31, PMID: , 10.1002/mnfr.201000121. [DOI] [PubMed] [Google Scholar]
- 9.Landrigan PJ, Goldman LR. 2011. Children’s vulnerability to toxic chemicals: a challenge and opportunity to strengthen health and environmental policy. Health Aff (Millwood) 30(5):842–850, PMID: , 10.1377/hlthaff.2011.0151. [DOI] [PubMed] [Google Scholar]
- 10.Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. . 2015. Executive summary to EDC-2: the Endocrine Society’s second Scientific Statement on endocrine-disrupting chemicals. Endocr Rev 36(6):593–602, PMID: , 10.1210/er.2015-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lunder S, Hovander L, Athanassiadis I, Bergman Å. 2010. Significantly higher polybrominated diphenyl ether levels in young U.S. children than in their mothers. Environ Sci Technol 44(13):5256–5262, PMID: , 10.1021/es1009357. [DOI] [PubMed] [Google Scholar]
- 12.Mamsen LS, Jönsson BAG, Lindh CH, Olesen RH, Larsen A, Ernst E, et al. . 2017. Concentration of perfluorinated compounds and cotinine in human foetal organs, placenta, and maternal plasma. Sci Total Environ 596–597:97–105, PMID: , 10.1016/j.scitotenv.2017.04.058. [DOI] [PubMed] [Google Scholar]
- 13.Kelley AS, Banker M, Goodrich JM, Dolinoy DC, Burant C, Domino SE, et al. . 2019. Early pregnancy exposure to endocrine disrupting chemical mixtures are associated with inflammatory changes in maternal and neonatal circulation. Sci Rep 9(1):5422, PMID: , 10.1038/s41598-019-41134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fudvoye J, Bourguignon JP, Parent AS. 2014. Endocrine-disrupting chemicals and human growth and maturation: a focus on early critical windows of exposure. Vitam Horm 94:1–25, PMID: , 10.1016/B978-0-12-800095-3.00001-8. [DOI] [PubMed] [Google Scholar]
- 15.Vrijheid M, Casas M, Gascon M, Valvi D, Nieuwenhuijsen M. 2016. Environmental pollutants and child health—a review of recent concerns. Int J Hyg Environ Health 219(4–5):331–342, PMID: , 10.1016/j.ijheh.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Braun JM. 2017. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol 13(3):161–173, PMID: , 10.1038/nrendo.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stratakis N, Rock S, La Merrill MA, Saez M, Robinson O, Fecht D, et al. . 2022. Prenatal exposure to persistent organic pollutants and childhood obesity: a systematic review and meta-analysis of human studies. Obes Rev 2(suppl 1):e13383, PMID: , 10.1111/obr.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohanto NC, Ito Y, Kato S, Kamijima M. 2021. Life-time environmental chemical exposure and obesity: review of epidemiological studies using human biomonitoring methods. Front Endocrinol (Lausanne) 12:778737, PMID: , 10.3389/fendo.2021.778737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Govarts E, Portengen L, Lambrechts N, Bruckers L, Den Hond E, Covaci A, et al. . 2020. Early-life exposure to multiple persistent organic pollutants and metals and birth weight: pooled analysis in four Flemish birth cohorts. Environ Int 145:106149, PMID: , 10.1016/j.envint.2020.106149. [DOI] [PubMed] [Google Scholar]
- 20.Agay-Shay K, Martinez D, Valvi D, Garcia-Esteban R, Basagaña X, Robinson O, et al. . 2015. Exposure to endocrine-disrupting chemicals during pregnancy and weight at 7 years of age: a multi-pollutant approach. Environ Health Perspect 123(10):1030–1037, PMID: , 10.1289/ehp.1409049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agier L, Basagaña X, Hernandez-Ferrer C, Maitre L, Tamayo Uria I, Urquiza J, et al. . 2020. Association between the pregnancy exposome and fetal growth. Int J Epidemiol 49(2):572–586, PMID: , 10.1093/ije/dyaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vrijheid M, Fossati S, Maitre L, Márquez S, Roumeliotaki T, Agier L, et al. . 2020. Early-life environmental exposures and childhood obesity: an exposome-wide approach. Environ Health Perspect 128(6):067009, PMID: , 10.1289/EHP5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang TC, Peterson KE, Meeker JD, Sánchez BN, Zhang Z, Cantoral A, et al. . 2018. Exposure to bisphenol A and phthalates metabolites in the third trimester of pregnancy and BMI trajectories. Pediatr Obes 13(9):550–557, PMID: , 10.1111/ijpo.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Svensson K, Tanner E, Gennings C, Lindh C, Kiviranta H, Wikström S, et al. . 2021. Prenatal exposures to mixtures of endocrine disrupting chemicals and children’s weight trajectory up to age 5.5 in the SELMA study. Sci Rep 11(1):11036, PMID: , 10.1038/s41598-021-89846-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iszatt N, Stigum H, Verner MA, White RA, Govarts E, Murinova LP, et al. . 2015. Prenatal and postnatal exposure to persistent organic pollutants and infant growth: a pooled analysis of seven European birth cohorts. Environ Health Perspect 123(7):730–736, PMID: , 10.1289/ehp.1308005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Botton J, Philippat C, Calafat AM, Carles S, Charles MA, Slama R, et al. . 2016. Phthalate pregnancy exposure and male offspring growth from the intra-uterine period to five years of age. Environ Res 151:601–609, PMID: , 10.1016/j.envres.2016.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heggeseth BC, Holland N, Eskenazi B, Kogut K, Harley KG. 2019. Heterogeneity in childhood body mass trajectories in relation to prenatal phthalate exposure. Environ Res 175:22–33, PMID: , 10.1016/j.envres.2019.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bier DM. 2008. Growth in the first two years of life. Nestle Nutr Workshop Ser Pediatr Program 61:135–144, PMID: , 10.1159/000113364. [DOI] [PubMed] [Google Scholar]
- 29.Woo Baidal JA, Locks LM, Cheng ER, Blake-Lamb TL, Perkins ME, Taveras EM. 2016. Risk factors for childhood obesity in the first 1,000 days: a systematic review. Am J Prev Med 50(6):761–779, PMID: , 10.1016/j.amepre.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Woo JG. 2019. Infant growth and long-term cardiometabolic health: a review of recent findings. Curr Nutr Rep 8(1):29–41, PMID: , 10.1007/s13668-019-0259-0. [DOI] [PubMed] [Google Scholar]
- 31.Druet C, Stettler N, Sharp S, Simmons RK, Cooper C, Smith GD, et al. . 2012. Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatr Perinat Epidemiol 26(1):19–26, PMID: , 10.1111/j.1365-3016.2011.01213.x. [DOI] [PubMed] [Google Scholar]
- 32.Zheng T, Zhang J, Sommer K, Bassig BA, Zhang X, Braun J, et al. . 2016. Effects of environmental exposures on fetal and childhood growth trajectories. Ann Glob Health 82(1):41–99, PMID: , 10.1016/j.aogh.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mook-Kanamori DO, Durmuş B, Sovio U, Hofman A, Raat H, Steegers EA, et al. . 2011. Fetal and infant growth and the risk of obesity during early childhood: the Generation R Study. Eur J Endocrinol 165(4):623–630, PMID: , 10.1530/EJE-11-0067. [DOI] [PubMed] [Google Scholar]
- 34.Oken E, Gillman MW. 2003. Fetal origins of obesity. Obes Res 11(4):496–506, PMID: , 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 35.Guxens M, Ballester F, Espada M, Fernández MF, Grimalt JO, Ibarluzea J, et al. . 2012. Cohort profile: the INMA—INfancia y Medio Ambiente—(Environment and Childhood) Project. Int J Epidemiol 41(4):930–940, PMID: , 10.1093/ije/dyr054. [DOI] [PubMed] [Google Scholar]
- 36.Goñi F, López R, Etxeandia A, Millán E, Amiano P. 2007. High throughput method for the determination of organochlorine pesticides and polychlorinated biphenyls in human serum. J Chromatogr B Anal Technol Biomed Life Sci 852(1–2):15–21, PMID: , 10.1016/j.jchromb.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 37.Grimalt JO, Howsam M, Carrizo D, Otero R, de Marchi MRR, Vizcaino E. 2010. Integrated analysis of halogenated organic pollutants in sub-millilitre volumes of venous and umbilical cord blood sera. Anal Bioanal Chem 396(6):2265–2272, PMID: , 10.1007/s00216-010-3460-y. [DOI] [PubMed] [Google Scholar]
- 38.Phillips DL, Pirkle JL, Burse VW, Bernert JT Jr, Henderson LO, Needham LL. 1989. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol 18(4):495–500, PMID: , 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- 39.Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Basterrechea M, Grimalt JO, et al. . 2015. Transfer of perfluoroalkyl substances from mother to fetus in a Spanish birth cohort. Environ Res 142:471–478, PMID: , 10.1016/j.envres.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 40.Sabaredzovic A, Sakhi AK, Brantsæter AL, Thomsen C. 2015. Determination of 12 urinary phthalate metabolites in Norwegian pregnant women by core–shell high performance liquid chromatography with on-line solid-phase extraction, column switching and tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 1002:343–352, PMID: , 10.1016/j.jchromb.2015.08.040. [DOI] [PubMed] [Google Scholar]
- 41.Valvi D, Casas M, Romaguera D, Monfort N, Ventura R, Martinez D, et al. . 2015. Prenatal phthalate exposure and childhood growth and blood pressure: evidence from the Spanish INMA-Sabadell birth cohort study. Environ Health Perspect 123(10):1022–1029, PMID: , 10.1289/ehp.1408887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vela-Soria F, Ballesteros O, Zafra-Gómez A, Ballesteros L, Navalón A. 2014. UHPLC-MS/MS method for the determination of bisphenol A and its chlorinated derivatives, bisphenol S, parabens, and benzophenones in human urine samples. Anal Bioanal Chem 406(15):3773–3785, PMID: , 10.1007/s00216-014-7785-9. [DOI] [PubMed] [Google Scholar]
- 43.Sakhi AK, Sabaredzovic A, Papadopoulou E, Cequier E, Thomsen C. 2018. Levels, variability and determinants of environmental phenols in pairs of Norwegian mothers and children. Environ Int 114:242–251, PMID: , 10.1016/j.envint.2018.02.037. [DOI] [PubMed] [Google Scholar]
- 44.Casas M, Valvi D, Luque N, Ballesteros-Gomez A, Carsin AE, Fernandez MF, et al. . 2013. Dietary and sociodemographic determinants of bisphenol A urine concentrations in pregnant women and children. Environ Int 56:10–18, PMID: , 10.1016/j.envint.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 45.Montazeri P, Vrijheid M, Martinez D, Basterrechea M, Fernandez-Somoano A, Guxens M, et al. . 2018. Maternal metabolic health parameters during pregnancy in relation to early childhood BMI trajectories. Obesity (Silver Spring) 26(3):588–596, PMID: , 10.1002/oby.22095. [DOI] [PubMed] [Google Scholar]
- 46.Montazeri P, Fossati S, Clemente DBP, Cirugeda L, Elosua R, Fernández-Barrés S, et al. . 2022. Early-childhood BMI trajectories in relation to preclinical cardiovascular measurements in adolescence. J Dev Orig Health Dis 13(3):322–329, PMID: , 10.1017/S2040174421000441. [DOI] [PubMed] [Google Scholar]
- 47.WHO. 2006. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva, Switzerland: WHO. https://www.who.int/tools/child-growth-standards [accessed 20 July 2021]. [Google Scholar]
- 48.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. 2007. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 85(9):660–667, PMID: , 10.2471/blt.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slining MM, Herring AH, Popkin BM, Mayer-Davis EJ, Adair LS. 2013. Infant BMI trajectories are associated with young adult body composition. J Dev Orig Health Dis 4(1):56–68, PMID: , 10.1017/S2040174412000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Domingo-Salvany A, Regidor E, Alonso J, Alvarez-Dardet C. 2000. Una propuesta de medida de la clase social. Aten primaria 25(5):350–363, PMID: , 10.1016/S0212-6567(00)78518-0. [DOI] [PubMed] [Google Scholar]
- 51.Chatzi L, Mendez M, Garcia R, Roumeliotaki T, Ibarluzea J, Tardón A, et al. . 2012. Mediterranean diet adherence during pregnancy and fetal growth: INMA (Spain) and RHEA (Greece) mother–child cohort studies. Br J Nutr 107(1):135–145, PMID: , 10.1017/S0007114511002625. [DOI] [PubMed] [Google Scholar]
- 52.van Buuren S, Groothuis-Oudshoorn K. 2011. mice: multivariate imputation by chained equations in R. J Stat Softw 45(3):1–67, 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 53.Montazeri P, Thomsen C, Casas M, de Bont J, Haug LS, Maitre L, et al. . 2019. Socioeconomic position and exposure to multiple environmental chemical contaminants in six European mother-child cohorts. Int J Hyg Environ Health 222(5):864–872, PMID: , 10.1016/j.ijheh.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Rossem L, Wijga AH, Brunekreef B, de Jongste JC, Kerkhof M, Postma DS, et al. . 2014. Overweight in infancy: which pre- and perinatal factors determine overweight persistence or reduction? A birth cohort followed for 11 years. Ann Nutr Metab 65(2–3):211–219, PMID: , 10.1159/000360305. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki K, Sato M, Zheng W, Shinohara R, Yokomichi H, Yamagata Z. 2015. Childhood growth trajectories according to combinations of pregestational weight status and maternal smoking during pregnancy: a multilevel analysis. PLoS One 10(2):e0118538, PMID: , 10.1371/journal.pone.0118538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haga C, Kondo N, Suzuki K, Sato M, Ando D, Yokomichi H, et al. . 2012. Developmental trajectories of body mass index among Japanese children and impact of maternal factors during pregnancy. PLoS One 7(12):e51896, PMID: , 10.1371/journal.pone.0051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fisher M, Arbuckle TE, Liang CL, LeBlanc A, Gaudreau E, Foster WG, et al. . 2016. Concentrations of persistent organic pollutants in maternal and cord blood from the Maternal–Infant Research on Environmental Chemicals (MIREC) cohort study. Environ Health 15(1):59, PMID: , 10.1186/s12940-016-0143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Martinez D, Ibarluzea J, et al. . 2016. Variability of perfluoroalkyl substance concentrations in pregnant women by socio-demographic and dietary factors in a Spanish birth cohort. Environ Int 92–93:357–365, PMID: , 10.1016/j.envint.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 59.Royston P, White IR. 2011. Multiple imputation by chained equations (MICE): implementation in Stata. J Stat Softw 45(4):1–20, 10.18637/jss.v045.i04. [DOI] [Google Scholar]
- 60.Colicino E, Pedretti NF, Busgang S, Gennings C. 2020. Per- and poly-fluoroalkyl substances and bone mineral density: results from the Bayesian weighted quantile sum regression. Environ Epidemiol 4(3):e092, PMID: , 10.1097/EE9.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Govarts E, Nieuwenhuijsen M, Schoeters G, Ballester F, Bloemen K, de Boer M, et al. . 2012. Birth weight and prenatal exposure to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE): a meta-analysis within 12 European birth cohorts. Environ Health Perspect 120(2):162–170, PMID: , 10.1289/ehp.1103767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valvi D, Mendez MA, Garcia-Esteban R, Ballester F, Ibarluzea J, Goñi F, et al. . 2014. Prenatal exposure to persistent organic pollutants and rapid weight gain and overweight in infancy.Obesity (Silver Spring) 22(2):488–496, PMID: , 10.1002/oby.20603. [DOI] [PubMed] [Google Scholar]
- 63.Liew Z, Goudarzi H, Oulhote Y. 2018. Developmental exposures to perfluoroalkyl substances (PFASs): an update of associated health outcomes. Curr Environ Health Rep 5(1):1–19, PMID: , 10.1007/s40572-018-0173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Braun JM. 2017. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol 13(3):161–173, PMID: , 10.1038/nrendo.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kahn LG, Philippat C, Nakayama SF, Slama R, Trasande L. 2020. Endocrine-disrupting chemicals: implications for human health. Lancet Diabetes Endocrinol 8(8):703–718, PMID: , 10.1016/S2213-8587(20)30129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dzierlenga MW, Crawford L, Longnecker MP. 2020. Birth weight and perfluorooctane sulfonic acid: a random-effects meta-regression analysis. Environ Epidemiol 4(3):e095, PMID: , 10.1097/EE9.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee DW, Lim HM, Lee JY, Min KB, Shin CH, Lee YA. 2022. Prenatal exposure to phthalate and decreased body mass index of children: a systematic review and meta-analysis. Sci Rep 12(1):8961, PMID: , 10.1038/s41598-022-13154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vrijheid M, Martinez D, Aguilera I, Ballester F, Basterrechea M, Esplugues A, et al. . 2012. Socioeconomic status and exposure to multiple environmental pollutants during pregnancy: evidence for environmental inequity? J Epidemiol Community Health 66(2):106–113, PMID: , 10.1136/jech.2010.117408. [DOI] [PubMed] [Google Scholar]
- 69.Nelson JW, Scammell MK, Hatch EE, Webster TF. 2012. Social disparities in exposures to bisphenol A and polyfluoroalkyl chemicals: a cross-sectional study within NHANES 2003–2006. Environ Health 11:10, PMID: , 10.1186/1476-069X-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Casas L, Fernández MF, Llop S, Guxens M, Ballester F, Olea N, et al. . 2011. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ Int 37(5):858–866, PMID: , 10.1016/j.envint.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 71.Tyrrell J, Melzer D, Henley W, Galloway TS, Osborne NJ. 2013. Associations between socioeconomic status and environmental toxicant concentrations in adults in the USA: NHANES 2001–2010. Environ Int 59:328–335, PMID: , 10.1016/j.envint.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 72.Thayer KA, Heindel JJ, Bucher JR, Gallo MA. 2012. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect 120(6):779–789, PMID: , 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Szilagyi JT, Avula V, Fry RC. 2020. Perfluoroalkyl substances (PFAS) and their effects on the placenta, pregnancy, and child development: a potential mechanistic role for placental peroxisome proliferator-activated receptors (PPARs). Curr Environ Health Rep 7(3):222–230, PMID: , 10.1007/s40572-020-00279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cano-Sancho G, Salmon AG, La Merrill MA. 2017. Association between exposure to p,p′-DDT and its metabolite p,p′-DDE with obesity: integrated systematic review and meta-analysis. Environ Health Perspect 125(9):096002, PMID: , 10.1289/EHP527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gingrich J, Ticiani E, Veiga-Lopez A. 2020. Placenta disrupted: endocrine disrupting chemicals and pregnancy. Trends Endocrinol Metab 31(7):508–524, PMID: , 10.1016/j.tem.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hutcheon JA, Chiolero A, Hanley JA. 2010. Random measurement error and regression dilution bias. BMJ 340:c2289, PMID: , 10.1136/bmj.c2289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.