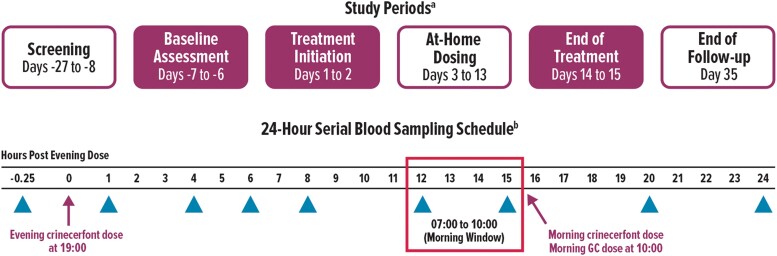
Figure 1.
Study design. aShaded boxes indicate overnight stay at study center for 24-hour serial blood sampling. bNo crinecerfont dose was administered on days −7/−6 (baseline visit). However, sample collection timepoints during this overnight stay were the same as days 1/2 and 14/15 (postbaseline visits). Triangles indicate timepoints when blood samples were collected. GC, glucocorticoid.