Figure 6. LPO neurons provide differential excitatory or inhibitory inputs to VTA-TH, VTA-VgluT2, and VTA-VGaT neurons.
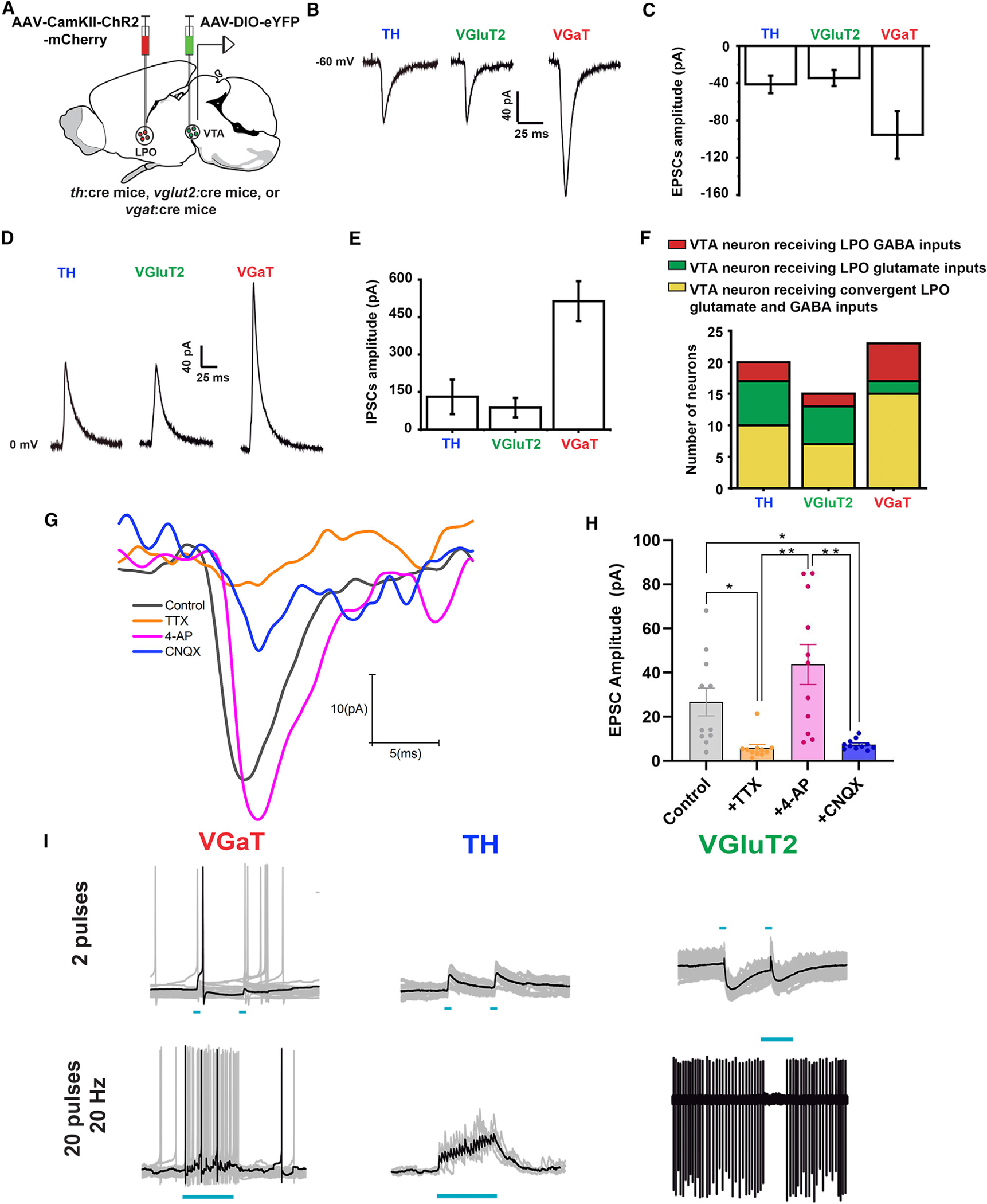
(A) Diagram of viral injection of AAV5-CAMKII-ChR2-mCherry into LPO and AAV5-DIO-eYFP into VTA of th:cre, vglut2:cre, or vgat:cre mice.
(B and C) VTA photostimulation of LPO inputs and voltage-clamp traces (B) and amplitude histogram (C) of isolated EPSCs recorded at −60 mV in VTA neurons (TH, VGluT2, and VGaT).
(D and E) VTA photostimulation of LPO inputs and voltage clamp traces (D) and amplitude histogram (E) of isolated IPSCs recorded at 0 mV in VTA neurons (TH, VGluT2, and VGaT).
(F) Histogram showing independent LPO excitatory, independent LPO inhibitory, and converging excitatory and inhibitory inputs on TH, VGluT2, and VGaT neurons.
G and H) Confirmation that LPO excitatory inputs to the VTA are monosynaptic. The perfusion of tetrodotoxin (TTX; 1 μM) decreased significantly (5.82 ± 1.61 pA) EPSCs (control, 26.68 ± 6.26 pA), and 4-AP (200 μM) recovered the amplitude of these currents (43.72 ± 9.09 pA). After the confirmation of the monosynaptic nature of the currents, the treatment with cyanquixaline (CNQX;10 μm) significantly decreased the glutamatergic currents (7.41 ± 0.71 pA) (treatment: F(3,30) = 14.08, p < 0.0001).
(I) Current clamp traces of VTA neurons. VTA photoactivation of LPO inputs activates VTA-TH neurons and inhibits VTA-VGaT neurons (20 Hz pulses; 2 pulses top or 20 pulses bottom). *p < 0.05; **p < 0.01; all data represent mean ± SEM.
