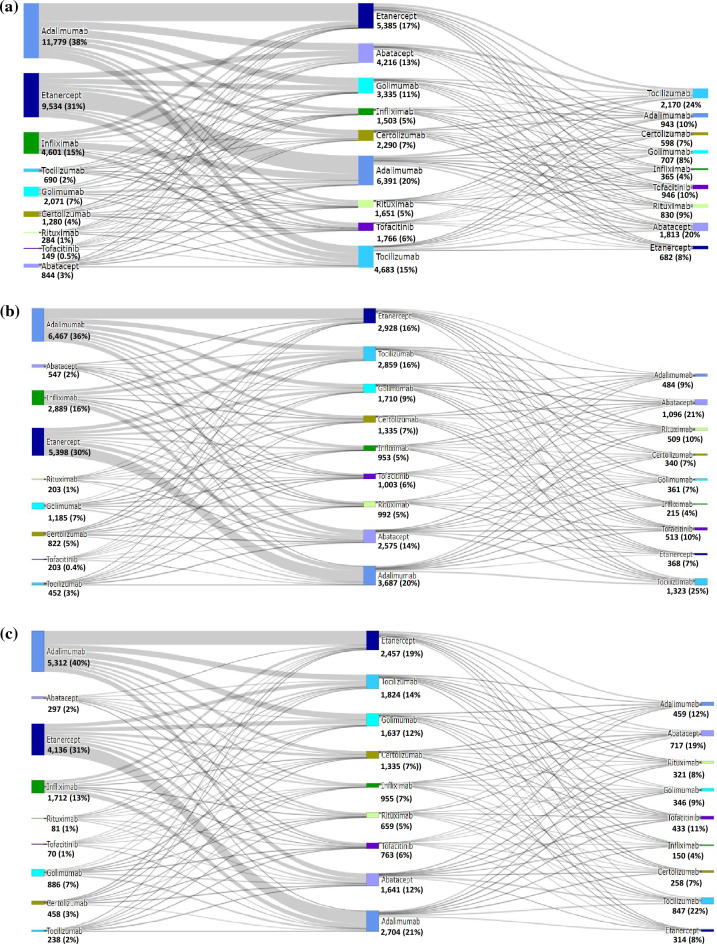
Figure 4.
(a) Sankey diagram depicting treatment sequencing among patients with rheumatoid arthritis initiating a b/tsDMARD who transition to a second-line, and/or third-line treatment (N = 31,232). b/tsDMARD biologic target synthetic disease modifying antirheumatic drug; tsDMARD limited to tofacitiniv at time of analysis; Sankey diagram limited to b/tsDMARDs. csDMARDs, conventional disease modifying antirheumatics, not shown and may be combination with b/tsDMARDs). (b) Sankey diagram of patients with rheumatoid arthritics initiating a b/tsDMARD who transition to a second-line, or third-line treatment (SUS-exclusive cohort) in DATASUS (N = 18,042). b/tsDMARD biologic target synthetic disease modifying antirheumatic. tsDMARD limited to tofacitinib at time of analysis;Sankey limited to b/tsDMARDs (csDMARD, conventional disease modifying antirheumatics, not shown and may be combination with b/tsDMARDs); SUS-exclusive are individuals, dependent on SUS for all healthcare resources. (c) Sankey diagram of patients with rheumatoid arthritis initiating a b/tsDMARD who transition to a second-line and/or third-line treatment (SUS+ private insurance cohort)in DATASUS (N = 13,190). b/tsDMARD biologic target synthetic disease modifying antirheumatic. tsDMARD limited to tofacitinib at time of analysis. Sankey limited to b/tsDMARDs (csDMARDs, conventional disease modifying antirheumatics, not shown and may be combination with b/tsDMARDs). SUS+ private insurance are individuals dependent on SUS only for prescription drug coverage.