Abstract
Background
Paraquat poisoning is one of the leading causes of fatal poisoning in many parts of the world, especially in agricultural countries. Its high toxicity even in small amounts causes rapid damage to multiple organs, especially the kidneys, lungs, and liver, mainly through free radical-mediated injury. As no specific antidote is yet available, early diagnosis and the importance of supportive therapy are critical parts of management. Some evidence suggests a survival benefit from using immunosuppressive drugs.
Case report
This case presentation concerns a 15-year-old boy from a village with a history of herbicide poisoning, later confirmed to be paraquat. Despite supportive therapy her condition continued to deteriorate with features of kidney and lung damage. The patient was then treated with methylprednisolone 500 mg daily for 5 days, along with other supportive care, and has made a remarkable recovery.
Conclusions
High efficacy as an herbicide, availability and low cost make paraquat an easy-to-encounter poison for suicidal or accidental use. Its high fatality calls for urgent and effective strategies to save lives. Methylprednisolone may play a role in its treatment.
Keywords: Pesticides, Herbicides, Paraquat poisoning, Methylprednisolone
Graphical Abstract

Highlights
-
•
Paraquat (PQ), a lethal herbicide, is taking human lives worldwide.
-
•
As it causes progressive damage to vital organs, frequent monitoring is essential to track the course of the disease.
-
•
As no antidote is yet available, methylprednisolone may play an important role in reversing the organ damage caused by PQ.
-
•
Raising public awareness and imposing a ban on PQ should be given immediate priority to save lives.
1. Background
Globally, pesticide poisoning is a leading cause of suicide related mortality, particularly in low and middle income countries [17]. Paraquat (PQ) is one of the lethal herbicides contributing to high mortality among pesticide related deaths [2]. Bangladesh, being an agrarian country, is likely to face a high incidence of PQ poisoning. Easy availability, cheap, unrestricted access to it, untrained applicator all together make this poisoning easier to happen. Till date there is a dearth of proven and curative treatments for this poisoning.
Even when ingested in a small amount, it is extremely toxic. Immediately after ingestion, the compound gets accumulated in different organs and the spectrum of complications like gastrointestinal injuries, respiratory failure, renal failure, hepatotoxicity, and lung fibrosis evolve over time. The suggested mechanism of toxicity of PQ is by production of reactive oxygen species which is the main culprit of cell damage through different pathways. Different treatment modalities have been applied with little evidence-based benefit so far. That’s why preventive strategy is the key to reducing the death toll from PQ poisoning.
Here we report a case of a 15-year-old boy who presented to us with a history of PQ poisoning 2 days back. Despite receiving supportive therapy including hemodialysis, he developed an acute kidney injury followed by a lung injury. Subsequently, administration of methylprednisolone resulted in significant clinical improvement and ultimately our patient survived.
2. Case description
A young boy of 15 years was presented to our hospital with a history of accidental ingestion of poison which he mentioned as weed killers, 2 days back. Immediately following ingestion, he was admitted to a nearby hospital from where he was referred to higher center for better management. Upon his admission, a detailed history regarding the type of poison, amount taken and current symptoms were noted. The poison identified was paraquat, a 20% W/V solution. He took about 3 tea spoonfuls. His current symptoms were excessive salivation, a burning sensation in the throat and chest pain. He also mentioned a decreased volume of urine for 1 day and on the 2nd day it was about 110 ml/24 h. During the course of his admission to our hospital he developed cough, hemoptysis, abdominal pain and progressive reduction of urine volume. He couldn’t take anything by mouth due to painful deglutition.
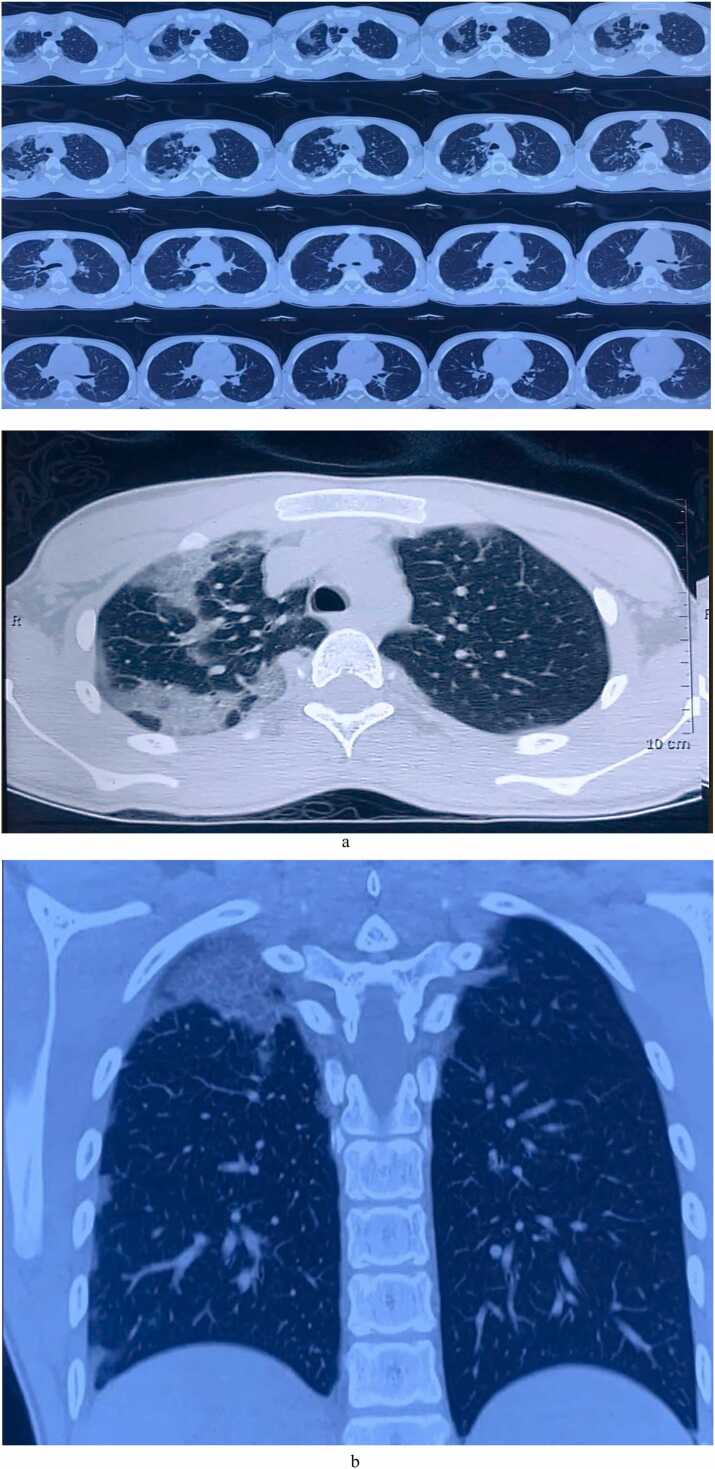
On admission, patients was conscious and well-oriented. His pulse was 86 beats/min, blood pressure 100/60 mm Hg, temperature 102°F and respiratory rate was slightly raised (22/min). His oxygen saturation was 95% at room air. The tongue was erythematous and a large confluent ulcer was found on its anterior part [Fig. 1]. Pupils were normal in size and chest examination findings were normal. Laboratory data [Table 1] found as follows: S. creatinine 6.8 mg/dl, Blood urea 55 mg/dl, ALT 20 U/L, S. sodium 141 mmol/l, S. potassium 3.9 mmol/l, chloride 100 mmol/l, Bicarbonate 16 mmol/l. Urine routine examinations revealed Albuminuria 2 + and protein-creatinine ratio was high (2.64). The chest X-Ray findings were unremarkable. Later on, high resolution computed tomography was performed on day 7 and revealed multifocal consolidation in both lungs [Fig. 2a and b].
Fig. 1.
A large coalescent ulcer over anterior parts of Tongue (taken on day 3).
Table 1.
Time trends of investigation.
| Name of investigations | Day 1 (August 2) |
Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 11 (August 12) |
|---|---|---|---|---|---|---|---|---|---|---|
| CBC | ||||||||||
| Hemoglobin (g/dl) | 14.2 | 12.6 | ||||||||
| WBC (×109/L) | 11.2 | 6.95 | ||||||||
| Neutrophil (%) | 74.5 | 85.3 | ||||||||
| Lymphocyte (%) | 19.9 | 12.4 | ||||||||
| Platelet (×109/L) | 165 | 175 | ||||||||
| ESR | 25 mm | 16 mm | ||||||||
| S. Creatinine (mg/dl) | 6.88 | 6.72 | 6.99 | 9.82 | 9 | 8.5 | 6.5 | 3.2 | 0.91 | |
| Blood urea (mg/dl) | 55 | 78 | 54 | |||||||
| ALT(IU/L) | 20 | 32 | ||||||||
| S. electrolyte | ||||||||||
| Sodium (mmol/l) | 141 | 146 | ||||||||
| potassium (mmol/l) | 3.9 | 3.3 | ||||||||
| chloride (mmol/l) | 100 | 105 | ||||||||
| Bicarbonate (mmol/l) | 16 | 26 | ||||||||
| Urine PCR (mg/mg) | 2.64 | |||||||||
| Urine R/M/E | Albumin + + RBC 2–3 cells/hpf |
Albumin-Nil, RBC- Nil |
||||||||
| Prothrombin time | patient- 17 s, control- 12 s |
|||||||||
| S. lipase | 66 | |||||||||
| Chest X-ray | NAD | |||||||||
| HRCT chest | Mild focal subpleural air space densities are seen in all segments of both lung (multifocal consolidation) | |||||||||
| USG of whole abdomen | NAD | |||||||||
Fig. 2.
a: HRCT showing focal subpleural air space densities in all segments of both lung (performed on day 7). b: HRCT showing air space densities and fibrosis in right upper lung field (performed on day 7).
He was initially treated with nothing per oral, as he couldn’t take an adequate diet due to dysphagia and started on IV fluid and other supportive measures. But his urine output was not improving and serum creatinine was rising. Then he was started on hemodialysis (HD). His condition was not improving after 2 days of HD. Then we gave an intravenous injection of methylprednisolone (MP). The dose was 500 mg daily. The patient's health improved after receiving the second dose of MP, as demonstrated by a progressive increase in urine volume and a reduction in the severity of symptoms [Table 2]. Intravenous MP was given for further three days. Since then the overall trend of the clinical course has been towards improvement. Serum creatinine also started to drop from 9.82 on day 4–0.91 on day 11.
Table 2.
Follow up chart during admission.
| Parameters | August 3 | August 4 | August 5 | August 6 | August 7 | August 8 | August 9 | August 10 | August 11 |
|---|---|---|---|---|---|---|---|---|---|
| Pulse | 62 | 70 | 66 | 68 | 74 | 80 | 78 | 78 | 76 |
| BP | 110/70 mmHg | 120/80 | 120/70 | 120/60 | 110/70 | 120/70 | 100/70 | 130/70 | 120/80 |
| Respiratory rate (/Min) | 24 | 26 | 26 | 26 | 25 | 24 | 22 | 20 | 19 |
| Temp | 103°F | 101°F | 99°F | 99°F | 99°F | 99°F | 99°F | 99°F | 99°F |
| Urine output (ml) | 110 | 400 | 400 | 900 | 1800 | 2100 | 2500 | 2700 | 2500 |
| SpO2 (at room air) | 98% | 97% | 95% | 94% | 94% | 94% | 95% | 96% | 98% |
He was discharged on day 11. At that time, his hemodynamic status and vital parameters were within normal limits.
2.1. Follow-up and outcomes
The patient was followed up after 3 weeks of discharge. He was free of any symptoms at that time. Renal function and liver function were normal.
3. Discussion
PQ poisoning is considered to be one of the fatal poisonings described in humans. Human poisoning from PQ was first reported in 1966, and in subsequent years more cases were affected with significant fatalities. According to WHO estimates, globally, more than 700,000 people die annually from suicide, and pesticide poisoning accounts for approximately 20% of these deaths [WHO]. Among the pesticides, paraquat poisoning seems to have the highest case fatality (50%–90%) [11].
PQ is a very effective herbicide. It is marketed as high concentration (usually 21%) for agricultural use and it needs to be diluted prior to use. But if this highly concentrated preparation is ingested, it is very lethal even in small quantities. It exerts its effect mostly by interference of the electron transport system and thus inhibits conversion of NADP to NADPH. The superoxide ion generated from this reaction leads to cell damage by lipid peroxidation of the cell membrane [4], [18].
Human poisoning mostly results from accidental or suicidal intent. Depending on the concentration of the formulation and amount ingested, clinical presentation may range from mild toxicity to severe toxicity which inevitably leads to death.
Initial symptoms are burning or pain in the oral cavity, oropharynx, throat or epigastrium, followed by vomiting. Later the tongue becomes erythematous, swollen, ulcerated with a yellowish necrotic base. Together these cause severe dysphagia which makes it almost impossible for the patient to eat by mouth. Patients at this stage are at risk for dehydration and prerenal acute kidney injury (AKI) due to decreased oral fluid intake.
In the next phase (within two to six days), renal and hepatocellular injury ensues. Renal injury mostly results from direct toxicity of PQ to renal tubular cells, primarily proximal tubular cells. Since the kidney is the major route of PQ excretion, this nephrotoxicity further complicates the situation by limiting PQ excretion from the body, ultimately leading to progressive renal failure.
The renal failure can be very rapid producing disproportionate rise in serum creatinine relative to blood urea (low Urea to Creatinine ratio) [3]. This rapid rise in serum creatinine probably reflects increased generation of creatine and creatinine to meet energy demand after significant oxidative stress [13]. We also noticed (day 1) lower ratio of Urea to creatinine (7.99) in this case.
Our patient had significant proteinuria reflecting PQ induced proximal tubular dysfunction. Similar findings were described in both animal and human models [10], [19].
Toxic hepatitis is quite common after PQ ingestion and mostly develops within the first week [20]. The spectrum of injuries is mostly mild and usually transient.
An autopsy study concluded that hepatic injury is biphasic, initially characterized by hepatocellular injury due to accumulation of parent compound. The second phase is characterized by cholangiocellular and cholestatic injury which probably results from toxic effect of unmetabolized PQ on biliary tree. Histological changes are mainly observed in the bile ducts [14].
In the final phase, the lungs are affected and this is main prognostic factors in PQ poisoning. PQ reaches its peak level 4 h after ingestion and then its concentration decline rapidly.
Intriguingly, PQ concentration in the lungs rises to several times the blood level during this period. Acute lung injury and ARDS developed in the early stage while lung fibrosis in the later stages [16]. Impaired gas exchange leads to hypoxemia before any radiological changes appeared. The radiological changes are usually bilateral; coarse, reticulonodular opacities, consolidation, sometimes ground glass opacities, and even pneumomediastinum have been reported [3]. In our case, HRCT done on 8th day reveals multifocal consolidation in both lungs and also fibrosis in right upper zone.
Management of PQ poisoning is mostly supportive because no specific antidote is yet available. Supportive therapy primarily focused on preventing GI absorption of ingested PQ by gastric lavage with activated charcoal or fuller's earth and removal of already absorbed products by hemodialysis or hemoperfusion. Since the immune system plays an important role in the pathogenesis here, various immunosuppressives have been tried in its management, especially methylprednisolone (MP) and cyclophosphamide. Although several observational studies have reported a small mortality benefit of immunosuppressive use [7], [8], [9] but systematic review found high heterogeneity, high risk of bias and weakness in the methodology of these studies, and the evidence was of low certainty [6]. In our case, methylprednisolone was given when hemodialysis failed to improve the condition and it worked. After 2 days of MP therapy his condition started to improve.
Considering the high fatality rate of PQ poisoning, different countries have adopted various strategies to reduce pesticide/paraquat related deaths.
Controls on imports, accessibility and even bans on these products have reduced suicides and pesticide-related deaths in several countries without negatively impacting agricultural production [1], [5], [12].
3.1. Limitations
We did not measure blood paraquat concentrations due to the unavailability of this assay in our hospital. If we could do that, we might find a correlation between blood PQ concentrations and the severity of organ damage.
4. Conclusion
The high toxicity of PQ poisoning is well established in the literature. But specific treatment is yet to be explored. In this case vignette, the patient developed early dysphagia, followed by progressive renal impairment and features of lung injury. The failure of supportive therapy prompted us to consider administration of methylprednisolone as a potential therapeutic approach. The patient's deteriorating condition then responded dramatically. This observation sheds light on the role of immunosuppressive in reducing or reversing PQ-induced organ damage. However, large well-designed clinical trials are needed to comment conclusively on this. Until then the government must take urgent steps to limit its use and accessibility and impose a ban on it and encourage the use of alternative herbicides.
4.1. Guidelines
The case report presentation followed CARE guidelines [15].
4.2. Learning points for clinicians
Physicians should be well-acquainted with the toxicity of paraquat. These patients should be observed for several days for the development of multi-organ dysfunction. Early diagnosis is essential to start supportive therapy as it can play an effective role. Although strong recommendations do not exist to date, the potentially beneficial role of methylprednisolone should not be overlooked.
4.3. Patient perspective
“The poison was kept in our house for use on agricultural land. One morning it was transferred to another bottle to make it ready for use. But I accidentally drink it mistaking it for water. After that, I had severe pain in my mouth and it was very difficult to eat anything. I was having trouble breathing. After receiving treatment in the hospital, my condition gradually improved. Now I can take my food and have no shortness of breath.”
Consent
Written informed consent was obtained from the patient's father for publication of this case report and the accompanying image. A copy of the written consent is available for review by the Editor-in-Chief upon request.
CRediT authorship contribution statement
Md Asaduzzaman: involved in patient’s care, Conceptualization, Data curation, literature reviews and manuscript writing. Soumitra Roy: involved in patient’s care, gathered the materials and were involved in Writing – original draft. Nibedita Das Pew: involved in patient’s care, gathered the materials and were involved in Writing – original draft. Anindya Deb Roy, Shahrin Kibria: gathered the materials, conducted a literature review. Ranjon Kumer Roy: interpreted patient data, assisted the other authors in reporting this case, edited the final draft. M.M. Jahangir Alam: interpreted patient data, assisted the other authors in reporting this case, edited the final draft. Shishir Ranjan Chakraborty: interpreted patient data, assisted the other authors in reporting this case, edited the final draft. All authors reviewed, edited, and approved the final version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful for the kind permission of the patient and her family to publish this case.
Handling Editor: Prof. L.H. Lash
Data availability
The authors do not have permission to share data.
References
- 1.Cha E.S., Chang S.-S., Gunnell D., Eddleston M., Khang Y.-H., Lee W.J. Impact of paraquat regulation on suicide in South Korea. Int. J. Epidemiol. 2016;45(2):470–479. doi: 10.1093/ije/dyv304. [DOI] [PubMed] [Google Scholar]
- 2.Dawson A.H., Eddleston M., Senarathna L., Mohamed F., Gawarammana I., Bowe S.J., et al. Acute human lethal toxicity of agricultural pesticides: a prospective cohort study. PLoS Med. 2010;7(10) doi: 10.1371/journal.pmed.1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinis-Oliveira R.J., Duarte J.A., Sánchez-Navarro A., Remião F., Bastos M.L., Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit. Rev. Toxicol. 2008;38(1):13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- 4.Dodge A. The mode of action of the bipyridylium herbicides, paraquat and diquat. Endeavour. 1971;30(111):130–135. doi: 10.1016/0160-9327(71)90039-1. [DOI] [PubMed] [Google Scholar]
- 5.Gunnell D., Fernando R., Hewagama M., Priyangika W.D.D., Konradsen F., Eddleston M. The impact of pesticide regulations on suicide in Sri Lanka. Int. J. Epidemiol. 2007;36(6):1235–1242. doi: 10.1093/ije/dym164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L.R., Chaudhary B., You C., Dennis J.A., Wakeford H. Glucocorticoid with cyclophosphamide for oral paraquat poisoning. Cochrane Libr. 2021;2021(6) doi: 10.1002/14651858.cd008084.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin J.-L., Lin-Tan D.-T., Chen K.-H., Huang W.-H., Hsu C.-W., Hsu H.-H., et al. Improved survival in severe paraquat poisoning with repeated pulse therapy of cyclophosphamide and steroids. Intensive Care Med. 2011;37(6) doi: 10.1007/s00134-010-2127-7. [DOI] [PubMed] [Google Scholar]
- 8.Lin J.-L., Leu M.-L., Liu Y.-C., Chen G.-H. A prospective clinical trial of pulse therapy with glucocorticoid and cyclophosphamide in moderate to severe paraquat-poisoned patients. Am. J. Respir. Crit. Care Med. 1999;159(2):357–360. doi: 10.1164/ajrccm.159.2.9803089. [DOI] [PubMed] [Google Scholar]
- 9.Lin J.L., Wei M.C., Liu Y.C. Pulse therapy with cyclophosphamide and methylprednisolone in patients with moderate to severe paraquat poisoning: a preliminary report. Thorax. 1996;51(7):661–663. doi: 10.1136/thx.51.7.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lock E.A., Ishmael J. The acute toxic effects of paraquat and diquat on the rat kidney. Toxicol. Appl. Pharmacol. 1979;50(1):67–76. doi: 10.1016/0041-008x(79)90493-9. [DOI] [PubMed] [Google Scholar]
- 11.Lock E.A., Wilks M.F. Handbook of Pesticide Toxicology. second ed. San Diego Academic Press; 2001. pp. 1559–1603. [Google Scholar]
- 12.Manuweera G., Eddleston M., Egodage S., Buckley N.A. Do targeted bans of insecticides to prevent deaths from self-poisoning result in reduced agricultural output? Environ. Health Perspect. 2008;116(4):492–495. doi: 10.1289/ehp.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohamed F., Endre Z., Jayamanne S., Pianta T., Peake P., Palangasinghe C., et al. Mechanisms underlying early rapid increases in creatinine in paraquat poisoning. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0122357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mullick F.G., Ishak K.G., Mahabir R., Stromeyer F.W. Hepatic injury associated with paraquat toxicity in humans. Liver. 2008;1(3):209–221. doi: 10.1111/j.1600-0676.1981.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 15.Riley D.S., Barber M.S., Kienle G.S., Aronson J.K., von Schoen-Angerer T., Tugwell P., et al. CARE guidelines for case reports: explanation and elaboration document. J. Clin. Epidemiol. 2017;89:218–235. doi: 10.1016/j.jclinepi.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Subbiah R., Tiwari R.R. The herbicide paraquat-induced molecular mechanisms in the development of acute lung injury and lung fibrosis. Crit. Rev. Toxicol. 2021;51(1):36–64. doi: 10.1080/10408444.2020.1864721. [DOI] [PubMed] [Google Scholar]
- 17.WHO, Suicide. Who.int. [cited 2023 Aug 29]. Available from: https://www.who.int/news-room/fact-sheets/detail/suicide.
- 18.Pasi A. Hans Huber; Bern: 1978. The Toxicity of Paraquat, Diquat and Morfamquat. [Google Scholar]
- 19.Vaziri N.D., Ness R.L., Fairshter R.D., Smith W.R., Rosen S.M. Nephrotoxicity of paraquat in man. Arch. Intern. Med. 1979;139(2):172–174. doi: 10.1001/archinte.139.2.172. [DOI] [PubMed] [Google Scholar]
- 20.Yang C.-J., Lin J.-L., Lin-Tan D.-T., Weng C.-H., Hsu C.-W., Lee S.-Y., et al. Spectrum of toxic hepatitis following intentional paraquat ingestion: analysis of 187 cases. Liver Int. 2012;32(9):1400–1406. doi: 10.1111/j.1478-3231.2012.02829.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.