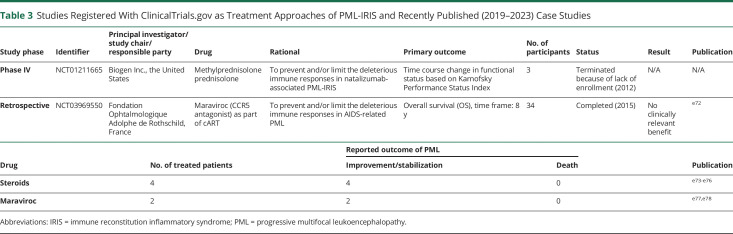
Table 3.
Studies Registered With ClinicalTrials.gov as Treatment Approaches of PML-IRIS and Recently Published (2019–2023) Case Studies
| Study phase | Identifier | Principal investigator/study chair/responsible party | Drug | Rational | Primary outcome | No. of participants | Status | Result | Publication |
| Phase IV | NCT01211665 | Biogen Inc., the United States | Methylprednisolone prednisolone | To prevent and/or limit the deleterious immune responses in natalizumab-associated PML-IRIS | Time course change in functional status based on Karnofsky Performance Status Index | 3 | Terminated because of lack of enrollment (2012) | N/A | N/A |
| Retrospective | NCT03969550 | Fondation Ophtalmologique Adolphe de Rothschild, France | Maraviroc (CCR5 antagonist) as part of cART | To prevent and/or limit the deleterious immune responses in AIDS-related PML | Overall survival (OS), time frame: 8 y | 34 | Completed (2015) | No clinically relevant benefit | e72 |
| Drug | No. of treated patients | Reported outcome of PML | Publication | |
| Improvement/stabilization | Death | |||
| Steroids | 4 | 4 | 0 | e73-e76 |
| Maraviroc | 2 | 2 | 0 | e77,e78 |
Abbreviations: IRIS = immune reconstitution inflammatory syndrome; PML = progressive multifocal leukoencephalopathy.