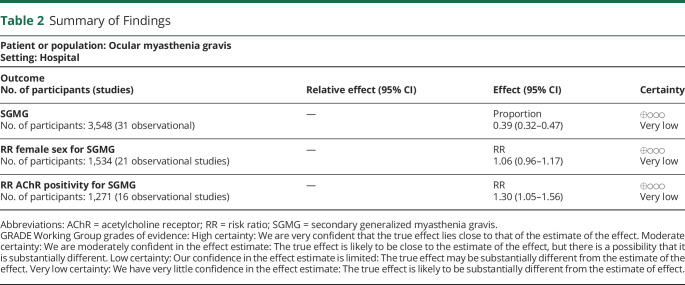
Table 2.
Summary of Findings
| Patient or population: Ocular myasthenia gravis Setting: Hospital | |||
| Outcome No. of participants (studies) |
Relative effect (95% CI) | Effect (95% CI) | Certainty |
| SGMG No. of participants: 3,548 (31 observational) |
— | Proportion 0.39 (0.32–0.47) |
⊕◯◯◯ Very low |
| RR female sex for SGMG No. of participants: 1,534 (21 observational studies) |
— | RR 1.06 (0.96–1.17) |
⊕◯◯◯ Very low |
| RR AChR positivity for SGMG No. of participants: 1,271 (16 observational studies) |
— | RR 1.30 (1.05–1.56) |
⊕◯◯◯ Very low |
Abbreviations: AChR = acetylcholine receptor; RR = risk ratio; SGMG = secondary generalized myasthenia gravis.
GRADE Working Group grades of evidence: High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.