Table 1.
Selected optimization experiments in the ruthenium-JOSIPHOS-catalyzed C-C coupling of gaseous allene 1a with alcohol 2a to form homoallylic alcohol 3a.a
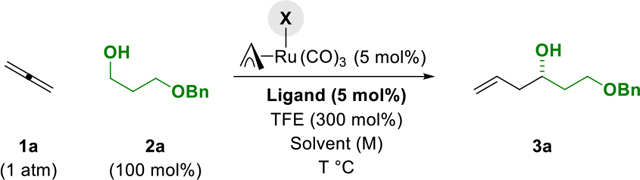
| |||||
|---|---|---|---|---|---|
| Entry | T °C | X | Ligand | Solvent | Yield, ee |
|
| |||||
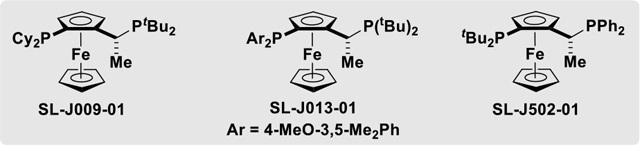
| 1 | 100 | I | SL-J009-01 | THF (0.5 M) | Trace |
| 2 | 110 | I | SL-J009-01 | THF (0.5 M) | 20%, 74% |
| 3 | 110 | I | SL-J013-01 | THF (0.5 M) | 65%, 69% |
| 4 | 110 | I | SL-J502-01 | THF (0.5 M) | 75%, 85% |
| 5 | 110 | I | SL-J502-01 | MTBE (0.5 M) | 78%, 87% |
| 6 | 110 | I | SL-J502-01 | DEE (0.5 M) | 88%, 88% |
|
|
110 | I | SL-J502-01 | DEE (0.1 M) | 86%, 90% |
| 8b | 110 | I | SL-J502-01 | DEE (0.1 M) | 42%, 90% |
| 9 | 110 | CI | SL-J502-01 | DEE (0.1 M) | Trace |
| 10 | 110 | Br | SL-J502-01 | DEE (0.1 M) | 29%, 75% |
| |||||
Yields of material isolated by silica gel chromatography. Enantioselectivities were determined by HPLC analysis.
TFE was omitted. See Supporting Information for further details.
