Abstract
OBJECTIVES:
ICU capacity strain is associated with worsened outcomes. Intermediate care units (IMCs) comprise one potential option to offload ICUs while providing appropriate care for intermediate acuity patients, but their impact on ICU capacity has not been thoroughly characterized. The aims of this study are to describe the creation of a medical-surgical IMC and assess how the IMC affected ICU capacity.
DESIGN:
Descriptive report with retrospective cohort review.
SETTING:
Six hundred seventy-three-bed tertiary care academic medical center with 77 ICU beds.
PATIENTS:
Adult inpatients who were admitted to the IMC.
INTERVENTIONS:
An interdisciplinary working group created an IMC which was located on a general ward. The IMC was staffed by hospitalists and surgeons and supported by critical care consultants. The initial maximum census was three, but this number increased to six in response to heightened critical care demand. IMC admission criteria also expanded to include advanced noninvasive respiratory support defined as patients requiring high-flow nasal cannula, noninvasive positive pressure ventilation, or mechanical ventilation in patients with tracheostomies.
MEASUREMENTS AND MAIN RESULTS:
The primary outcome entailed the number of ICU bed-days saved. Adverse outcomes, including ICU transfer, intubation, and death, were also recorded. From August 2021 to July 2022, 230 patients were admitted to the IMC. The most frequent IMC indications were respiratory support for medical patients and post-operative care for surgical patients. A total of 1023 ICU bed-days were made available. Most patients were discharged from the IMC to a general ward, while 8% of all patients required transfer to an ICU within 48 hours of admission. Intubation (2%) and death (1%) occurred infrequently within 48 hours of admission. Respiratory support was the indication associated with the most ICU transfers.
CONCLUSIONS:
Despite a modest daily census, an IMC generated substantial ICU bed capacity during a time of peak critical care demand.
Keywords: critical care, intensive care unit capacity, intermediate care, respiratory failure
KEY POINTS
Question: How much ICU capacity did a newly opened mixed patient intermediate care unit generate?
Findings: With a maximum census ranging from three to six beds and admission criteria that included advanced noninvasive respiratory support, a mixed medical-surgical IMC situated within a general ward generated substantial critical care capacity. Adverse events within 48 hours of IMC admission were infrequent and more common in the medical population than the surgical population.
Meaning: This study suggests that an IMC can offer an effective means of increasing ICU bed capacity.
Heightened critical care demand and ICU capacity strain are associated with worsened mortality (1, 2). Drivers of capacity strain include patient acuity, patient census, and admissions (3). Prior studies have estimated that 19–35% of ICU admissions are associated with sufficiently low risk of negative outcomes that they may be safely admitted to lower levels of care (3, 4).
Intermediate care units (IMCs) are defined as inpatient medical units designed to care for a diverse range of patients who require a higher level of monitoring, frequent nursing care, and/or specific therapies that cannot be provided on a general ward but who simultaneously do not require ICU level care (5, 6). There is substantial heterogeneity in IMC nomenclature, admission criteria, staffing, and patient composition (7).
Pre-pandemic data suggests that IMCs improve ICU bed utilization and patient outcomes (8, 9). The specific impact that opening an IMC to care for intermediate acuity patients has on ICU bed capacity, however, has not been well characterized. Additionally, the COVID-19 pandemic generated an overwhelming need for critical care resources that stressed ICU capacity and contributed to worsened outcomes (10). Data describing the utilization of IMCs during the pandemic is limited and focuses on patient outcomes more so than their impact on ICU occupancy and patient flow (11, 12).
In response to longstanding critical care needs that were exacerbated during the pandemic, our institution opened an IMC on a general ward to augment ICU capacity. The aims of this study are to describe the creation and expansion of this medical-surgical IMC and establish the number of ICU bed-days saved along with other outcomes associated with patient care in the IMC. This study will also provide a local baseline of outcomes—particularly for patients with respiratory failure—for future comparison while offering guidance for how to launch an IMC.
METHODS
Design
This is a descriptive study that was deemed exempt by the Beth Israel Deaconess Medical Center (BIDMC) Institutional Review Board. The IMC initiative comprised an institutional quality improvement project such that the results are reported according to the Standards for QUality Improvement Reporting Excellence 2.0 guidelines (13).
Setting
BIDMC is a 673-bed tertiary care academic medical center with 77 ICU beds divided among medical, cardiac, surgical, trauma, and neurologic specialties. Between August 2021 and July 2022, approximately 37,000 patients were admitted to BIDMC. The median (interquartile range) ICU occupancy during this period was 84.5% (83.5–87.9%). The percent of ICU beds used by patients with COVID-19 (number of COVID-19 patients in the ICU patients divided by the total number of ICU beds) (1) was 2–23% with the peak occurring in January 2022.
IMC Model Development and Iteration
An interprofessional IMC working group spanning critical care, surgery, hospital medicine, nursing, and respiratory therapy was convened in late 2020 to establish the staffing model, determine inclusion/exclusion criteria, coordinate staff training, and oversee growth of the IMC.
The initial iteration of the IMC was opened in August 2021. Because constructing an entirely new physical space for the IMC was not feasible, IMC patients were admitted to a 16-bed general ward that was adapted to meet IMC patient needs. IMC patients were assigned specific rooms located in one section of the ward that already had the necessary structural capabilities (e.g., wall suction and oxygen ports). The remainder of the unit continued to care for general medical-surgical patients, and the IMC rooms could be used to care for general ward patients if not occupied by an IMC patient.
Figure 1 describes IMC staffing compared with the ICU and general wards. As a mixed medical-surgical IMC, hospitalists attended on medical patients while surgical patients were cared for by the specific surgical service associated with the patient. Bedside nursing care was provided by nurses trained in intermediate medical-surgical care with a nurse-to-patient ratio of 1:3–4. For comparison, the nurse-to-patient ratio for general medical-surgical patients was 1:4 during the day and 1:5–6 at night. Respiratory care was provided by two respiratory therapists who were also responsible for a 12-bed ICU, a post-anesthesia care unit (PACU), and a several medical-surgical wards located in the same building as the IMC.
Figure 1.
Unit staffing and patient census comparison, August 2021 to July 2022. IMC = intermediate care unit.
Some of the general ward nurses underwent training led by IMC nurse specialists who crafted and delivered a curriculum based on IMC admission criteria. All IMC patients were usually assigned to one nurse each shift; although staffing and patient care needs sometimes required an IMC nurse to simultaneously care for IMC and general ward patients. For example, a patient de-escalating from IMC to general ward status could remain assigned to the same nurse to facilitate continuity. These logistics were managed by the unit’s resource nurse who coordinated nursing assignments for both general ward and IMC patients.
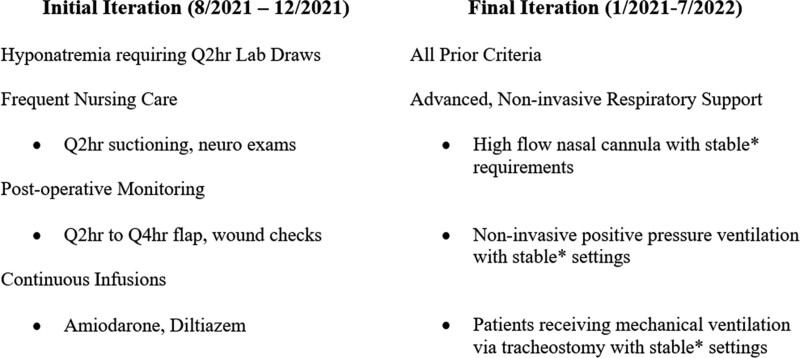
To admit a patient to the IMC, referring providers contacted a triage intensivist who reviewed the case. Admission criteria were initially limited to patients requiring frequent nursing care (e.g., suctioning every 2 hr, postoperative wound checks every 2 hr), frequent serum sodium monitoring, and continuous infusions of diltiazem or amiodarone (Fig. 2). No specific parameters related to vital signs, laboratories, or other data were established to allow flexibility in IMC admissions, which were evaluated comprehensively by the triage intensivist who conferred with IMC resource nurse and hospitalist. Before the IMC, the indications present in Figure 2 were otherwise not permitted on general wards, such that patients fitting these indications would require ICU admission until these needs resolved. IMC patients were evaluated for de-escalation to the general wards each day, with de-escalation dependent upon resolution of the IMC admission indication.
Figure 2.
Intermediate care unit (IMC) admission criteria, August 2021 to July 2022. *Stability was defined as consistent or improving settings, including Fio2, flow, and positive end-expiratory pressure for at least 24 hr before IMC transfer. Q2hr = every 2 hr, Q4hr = every 4 hr.
Critical care consultation occurred within 24 hours for all patients admitted for medical indications and was offered to all surgical patients. Patients were initially triaged to the IMC exclusively from an ICU or PACU. After several months, patients from the emergency department (ED) and general wards were also accepted for admission in a “step-up” fashion.
The initial maximum census was three IMC beds. The census and admission criteria evolved over time under the guidance of the IMC Working Group. At the end of 2021, BIDMC witnessed a surge of ICU volume in the context of the Omicron variant, prompting expansion of the IMC to a maximum census of six. The inclusion criteria were also expanded to include patients requiring stable amounts of advanced respiratory support with high-flow nasal cannula, newly initiated noninvasive positive pressure ventilation, or mechanical ventilation via tracheostomy (Fig. 2). Stability was defined as consistent or improving settings, such as Fio2, flow, and positive end-expiratory pressure for at least 24 hours before IMC transfer. As with other IMC admission criteria, clinical data were wholistically assessed by the triage intensivist with input from IMC nursing and hospitalists, but there were no specific thresholds for admission.
De-escalation from the IMC to the general ward for patients requiring respiratory support was dependent upon resolution of the patient’s need for high-flow nasal cannula or noninvasive positive pressure support. Patients requiring mechanical ventilation via tracheostomy were not eligible for de-escalation to a general ward and remained in the IMC until discharge.
Measurements
Patient demographics as well as diagnosis, location before IMC admission, location after discharge from IMC, and IMC readmissions were recorded.
The primary outcome was the number of ICU bed-days saved. This measure was calculated using IMC length of stay, which was determined for each patient and then summated. Before creation of the IMC, patients qualifying for the IMC would have been admitted to an ICU such that the net IMC length of stay approximates the ICU capacity made available. Each patient’s hospital length of stay was also recorded.
Adverse outcomes recorded were ICU transfer, intubation, and death occurring within 48 hours of IMC admission as well as for the entire hospital stay after IMC admission (i.e., any time after IMC admission but before hospital discharge). Identifying that some patients’ illness would progress over time regardless of level of care, the author group (E.A.K., E.K., J.Y., M.M.H.) chose a 48-hour window to measure adverse events and identify patients that could have been triaged to or remained in an ICU. Under triage correlates with unintended ICU transfers, which in turn are associated with excess length of stay and mortality (14, 15). Delays in ICU admission after initial referral are also associated with increased mortality (16). While prior IMC literature typically focuses on the 24-hour period after admission (3, 14, 15), we selected a longer time frame to capture more potential adverse outcomes and safety concerns, which was a priority at our institution.
All measures were reported for the entire study. Two authors (E.A.K., E.K.) extracted patient data manually from the electronic health records. Discrepancies in data extraction were reviewed and adjudicated by a third author (M.M.H.). Data were analyzed using R (Vienna, Austria) (17). Statistical significance of differences among subgroups for numeric data were assessed using the Welch two-sample t test or in the case of lengths of stays, the Mann-Whitney U test. The association between patient subgroups and categorical variables were tested using Pearson chi-square or Fisher exact test. Statistical significance was assessed at the 0.05 level.
RESULTS
Study Population, Origin, and Destination
From August 2021 to July 2022, 230 patients were admitted to the IMC with more medical patients (131) than surgical patients (89). Table 1 summarizes patient demographics and IMC admission criteria. The average age was 62, 55% were female, 32% identified as non-White, and 17% were positive for COVID-19. Within the subgroups, the most frequent indication was advanced respiratory support for medical patients (51%) and postoperative monitoring for surgical patients (77%). Compared with the surgical cohort, medical patients were significantly older and were more likely to have COVID-19 upon admission.
TABLE 1.
Intermediate Care Unit Patient Demographics and Admission Indications, August 2021 to July 2022
| Characteristic | Medical, n = 141 | Surgical, n = 89 | All, n = 230 | p |
|---|---|---|---|---|
| Age, average | 64 | 56 | 61 | < 0.001 |
| Sex, % female | 66 (47%) | 60 (67%) | 126 (55%) | 0.003 |
| Race/ethnicitya | 0.19 | |||
| Non-White | 51 (36%) | 22 (25%) | 73 (32%) | |
| White | 79 (56%) | 58 (65%) | 137 (60%) | |
| Unknown | 11 (7.8%) | 9 (10%) | 20 (8.7%) | |
| COVID+ on admissiona | 38 (27%) | 1 (1%) | 39 (17%) | < 0.001 |
| Intermediate care unit indicationa | < 0.001 | |||
| Advanced, noninvasive respiratory support | 72 (51%) | 1 (1%) | 73 (32%) | |
| Continuous infusion (amiodarone, diltiazem) | 3 (2%) | 2 (2%) | 5 (2%) | |
| Serum sodium monitoring | 24 (17%) | 0 (0%) | 24 (10%) | |
| Possible inappropriate admission | 5 (3%) | 0 (0%) | 5 (2%) | |
| Postoperative monitoring (e.g., wound/flap checks) | 1 (1%) | 68 (77%) | 69 (30%) | |
| Nursing care (e.g., suctioning) | 35 (25%) | 17 (19%) | 52 (23%) | |
| Other | 1 (1%) | 1 (1%) | 2 (1%) | |
n (%).
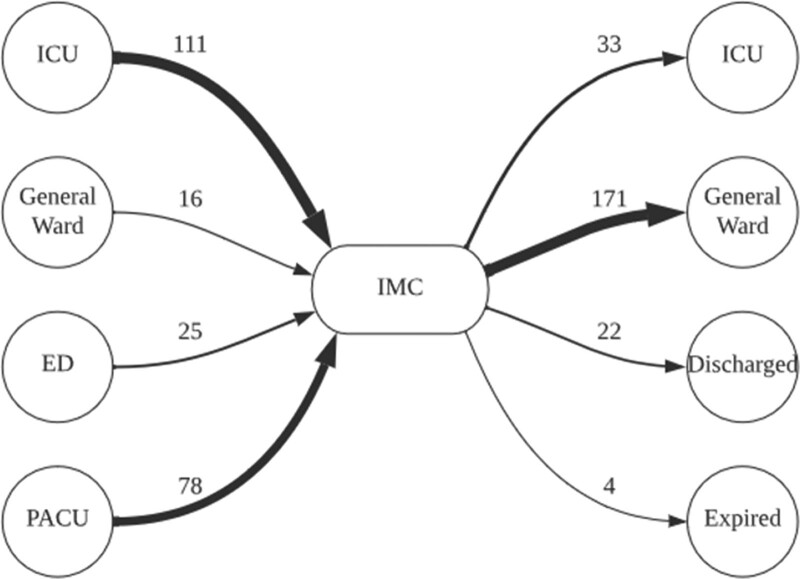
Figure 3 outlines origins and destinations for patients admitted to the IMC. Most IMC patients were admitted in a step-down fashion from ICUs and the PACU, with only 18% admitted in a step-up fashion from the ED or a general ward. Readmission to the IMC occurred in 8% of patients. The most common destination following the IMC for all patients was a general ward (74%).The median length of IMC stay and median hospital length of stay were significantly higher for medical patients as compared with surgical patients (3 vs. 2; p < 0.01 and 19 vs. 5; p < 0.01).
Figure 3.
Origins and destinations of intermediate care unit (IMC) patients, August 2021 to July 2022. ED = emergency department, PACU = post-anesthesia care unit.
Table 2 summarizes outcomes and adverse events. A total of 1023 ICU bed-days were made available by admitting patients to the IMC who would have otherwise occupied an ICU bed, with 852 created by the medical cohort and 171 by the surgical cohort. The IMCs average daily census was 2.8, implying that 2.8 ICU beds were made available per day.
TABLE 2.
Intermediate Care Unit Outcomes and Adverse Events, August 2021 to July 2022
| Outcome or Adverse Event | Medical, n = 141 | Surgical, n = 89 | All, n = 230 | p a |
|---|---|---|---|---|
| Outcome | ||||
| ICU bed days savedb | 852 (552–1255) | 171 (141–207) | 1023 (704–1431) | - |
| IMC LOSc | 3 (1–6) | 2 (1–2) | 2 (1–4) | < 0.01 |
| Hospital LOSd | 19 (8–40) | 5 (3–10) | 11 (5–28) | < 0.01 |
| Adverse eventd,e | ||||
| ICU transfer < 48 hr | 15 (11%) | 3 (3%) | 18 (8%) | 0.08 |
| ICU transfer after IMC admit | 34 (24%) | 3 (3%) | 37 (16%) | < 0.01 |
| Intubation < 48 hr | 5 (3%) | 0 (0%) | 5 (2%) | 0.16 |
| Intubation after IMC admit | 10 (7%) | 1 (1%) | 11 (5%) | 0.05 |
| Death < 48 hr | 3 (2%) | 0 (0%) | 3 (1%) | 0.29 |
| Death after IMC admit | 24 (17%) | 0 (0%) | 24 (10%) | < 0.01 |
IMC = intermediate care unit, LOS = length of stay.
Fisher exact test.
Sum (nonparametric Bootstrapped 95% CI).
Median (Q1–Q3).
n (%).
“After IMC Admit” refers to an event occurring at any point after the patient was admitted to the IMC before their discharge from the hospital.
Three IMC encounters—all of which were medical patients with an indication of advanced respiratory support—lasted longer than 50 days and accounted for 30% of all ICU bed-days saved. The duration of these encounters was predominantly driven by challenges regarding placement after discharge, particularly with locating post-acute care facilities that could accommodate patients receiving intermittent dialysis and mechanical ventilation via tracheostomy.
Regarding adverse events, 8% of all patients required ICU transfer, 2% required intubation, and 1% expired within 48 hours of IMC admission. A higher percentage of medical patients required ICU transfer within 48 hours as compared with surgical patients, but the comparison did not reach the threshold of statistical significance (11% vs. 3%; p = 0.11). The majority of patients (66%) who escalated to the ICU within 48 hours had been admitted to the IMC for advanced respiratory support. Although small sample sizes in the ED and general ward subcategories limited robust statistical comparisons, adverse events within 48 hours of IMC admission were grossly comparable across different origin locations (Table 3).
TABLE 3.
Adverse Events Within 48 Hours of Intermediate Care Unit Admission According to Origin Location
| Origin Before Intermediate Care Unit | n | ICU Transfer, n (%) | Intubation, n (%) | Death, n (%) |
|---|---|---|---|---|
| Emergency department | 25 | 1 (4) | 0 (0) | 0 (0) |
| General ward | 15 | 2 (13) | 1 (7) | 2 (13) |
| ICU | 111 | 15 (14) | 3 (3) | 1 (1) |
| Other | 1 | 0 (0) | 0 (0) | 0 (0) |
| Post-anesthesia care unit | 78 | 0 (0) | 0 (0) | 0 (0) |
Regarding adverse outcomes at any point after IMC transfer, ICU transfers (24% vs. 3%; p < 0.01) and death (17% vs. 0%; p < 0.01) were significantly more common in the medical population compared with the surgical population. The number patients requiring intubation was higher among medical patients but did not meet the threshold of statistical significance (7.1% vs. 1.1%; p = 0.05).
DISCUSSION
In summary, we describe the 1-year experience of a mixed medical-surgical IMC created during the COVID-19 pandemic. Over the course of 12 months with a census that expanded from three to six, a total of 230 patients were admitted to the IMC, and 1023 ICU bed-days were generated. The majority of IMC patients were transferred to a general ward following a median length of stay of 2 days, whereas 8% of patients required transfer to an ICU within 48 hours of IMC admission.
With a small census and nursing ratio comparable to previously published descriptions (7), our hospital’s IMC generated ICU capacity by adapting and upstaffing a preexisting medical-surgical ward. Assuming that IMC patients would have otherwise been admitted to the ICU, the average daily census of the IMC of 2.8 beds from August 2021 to July 2022 equates to 3.6% of our institution’s ICU occupancy. These improvements in ICU capacity align with prior evidence showing that IMCs improve ICU bed utilization (8, 9). For some patients, situating the IMC on a general ward also allowed for facile de-escalations of care that promoted continuity and averted transfers to other wards. Although the IMCs maximum potential census was six, at times IMC beds could be allocated to general ward patients based on institutional needs to reduce wait times in the ED or PACU.
The flexibility of this model must be weighed against the need to train up and support IMC staff, the challenge of optimally allocating IMC vs. general ward beds, and the demands of providing frequent monitoring and interventions to intermediate acuity patients while simultaneously caring for a separate population of general medical-surgical patients located on the same ward. This model was dependent upon tremendous upfront and daily efforts particularly from the nursing staff. The reduction of ICU capacity issues and the associated safety risks—recognized priorities throughout our institution—were important considerations for both frontline staff and institutional leadership, who sponsored the operationalization of the new unit. Finally, the creation of an interprofessional working group, which included nurses, respiratory therapists, physicians, and hospital leadership, was key to establishing a unified approach for how and when the IMC would be expanded—particularly the addition of patients requiring advanced respiratory support.
Although necessary to meet rising capacity issues, the introduction of advanced respiratory support as an admission criterion was known to carry risk. Intubation and death within 48 hours of IMC admission occurred rarely. However, patients requiring advanced respiratory support constituted the largest group of patients necessitating transfer to the ICU within 48 hours of IMC admission. Determining which IMC patients will require ICU level of care can be difficult, even with prediction tools (18). At our hospital, efforts are ongoing to update triage criteria and determine which advanced respiratory failure patients are the most appropriate for an IMC.
The rate of ICU transfer, intubation, and death at any point after IMC admission and before hospital discharge were significantly higher in the medical population than the surgical population, with an overall hospital mortality of 10% for patients admitted to the IMC (Table 2). Adverse outcomes at any point after IMC admission may reflect progression of intermediate acuity conditions despite appropriate management and, if approached safely, do not necessarily imply suboptimal triage.
Outcomes from this IMC are within the range of prior IMC literature before and during the pandemic (11, 12, 19, 20). Two studies have indicated that 16–39% of patients admitted to an IMC for respiratory support during the pandemic required transfer to an ICU and up to 13% of IMC patients expired during their IMC admission (11, 12). In the study conducted by Matute-Villacís et al (11), 26% of patients who were admitted to the IMC had a do-not-resuscitate order, which constituted a contraindication to ICU admission. In the study by Grosgurin et al (12), patients who were excluded from ICU admission due to de-escalation in care were excluded from the analysis.
Our IMC was designed for patients with conditions expected to resolve over a brief period. As the IMC inclusion criteria expanded and institutional demands evolved, however, the IMC took on several long-stay patients whose length of stay exceeded 50 days. These patients were medically complex with advanced respiratory needs who required frequent suctioning and/or overnight ventilator use while awaiting safe disposition. These needs limited the ability to safely care for these patients outside of the ICU or IMC and required IMC staff to develop new skills and knowledge. Patients with prolonged hospitalizations comprise a vulnerable population that face numerous barriers to discharge and manifest significant cost and resource utilization issues for hospital systems (21, 22). Although not its intended focus, the IMC nonetheless served a crucial role caring for long-stay, intermediate acuity patients outside of an ICU where they otherwise would have been admitted. Institutions considering the creation or expansion of an IMC may benefit from considering long-stay patients as part of IMC design, staff training, and capacity projections.
Limitations
There are several important limitations to our study. First, our IMC was created and adapted in real time to meet evolving capacity demands during the COVID-19 pandemic, which precluded the creation of a control group. In addition to multiple confounding factors, including other institutional adaptations to meet capacity demands and limitations in staffing, the absence of a medical-surgical IMC before the pandemic made additional analyses, including comparisons to pre-pandemic data infeasible.
Second, bed tracking software at our institution was limited in its ability to track the IMC census. Determining IMC census necessitated manual review of electronic health records, which may have led to an underestimation of IMC patient volume. Additionally, the bed tracking software calculates unit censes at specific times during the day, which may lead to a misrepresentation of average census of the ICU.
Third, the primary outcome of ICU bed days was determined based on measurement of IMC length of stay, which assumes that IMC length of stay directly equates to ICU length of stay. This assumption was thought to be justified because before creation of the IMC at BIDMC, patients with IMC indications would have otherwise been admitted to an ICU. However, there are likely some discrepancies between IMC length of stay and the theoretical ICU length of stay such that this approximation is not exact.
Fourth, the single center nature may limit this study’s generalizability. Our IMC benefited greatly from local interprofessional expertise and needs may vary between institutions. Certain aspects of our experience, including the use of ICU capacity parameters to guide IMC expansion and the operationalization of an IMC without relying on a new physical space may still inform IMC formation in other contexts.
Future Directions
To our knowledge, this is the first study describing an IMC staffed by hospitalists featuring obligate critical care consultation for medical patients. While ICU consults are associated with improved ICU mortality and readmission rates, they frequently occur in emergent scenarios, such as rapid responses rather than in an expected, proactive fashion (23, 24). Furthermore, the optimal model of care for IMC staffing has not been defined, with prior data suggesting that progressive care units staffed by hospitalists and intensivists experience similar patient outcomes (25). We hypothesize that intensivist consultation promoted positive outcomes, with unpublished survey data gathered from our IMC hospitalists indicating that intensivist input correlated with management changes for several indications—particularly respiratory support. A specific analysis of obligate critical care consults for intermediate care patients may yield additional insight into their potential impact on patient outcomes.
CONCLUSIONS
In conclusion, a mixed medical-surgical IMC staffed by hospitalists and surgeons and supported by critical care consultation generated robust ICU bed capacity despite a modest daily census. Adverse events within 48 hours of admission occurred infrequently and were driven by patients admitted for advanced respiratory support.
Footnotes
Drs. Yang and Hayes are co-senior authors.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Lapichino G, Gattinoni L, Radrizzani D, et al. : Volume of activity and occupancy rate in intensive care units. Association with mortality. Intensive Care Med 2004; 30:290–297 [DOI] [PubMed] [Google Scholar]
- 2.Gabler NB, Ratcliffe SJ, Wagner J, et al. : Mortality among patients admitted to strained intensive care units. Am J Respir Crit Care Med 2013; 188:800–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmerman JE, Wagner DP, Knaus WA, et al. : The use of risk predictions to identify candidates for intermediate care units. Implications for intensive care utilization and cost. Chest 1995; 108:490–499 [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal GE, Sirio CA, Shepardson LB, et al. : Use of intensive care units for patients with low severity of illness. Arch Intern Med 1998; 158:1144–1151 [DOI] [PubMed] [Google Scholar]
- 5.Nasraway SA, Cohen IL, Dennis RC, et al. : Guidelines on admission and discharge for adult intermediate care units. American College of Critical Care Medicine of the Society of Critical Care Medicine. Crit Care Med 1998; 26:607–610 [DOI] [PubMed] [Google Scholar]
- 6.Prin M, Wunsch H: The role of stepdown beds in hospital care. Am J Respir Crit Care Med 2014; 190:1210–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plate JDJ, Leenen LPH, Houwert M, et al. : Utilisation of intermediate care units: A systematic review. Crit Care Res Pract 2017; 2017:8038460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solberg BC, Dirksen CD, Nieman FH, et al. : Introducing an integrated intermediate care unit improves ICU utilization: A prospective intervention study. BMC Anesthesiol 2014; 14:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capuzzo M, Volta C, Tassinati T, et al. ; Working Group on Health Economics of the European Society of Intensive Care Medicine: Hospital mortality of adults admitted to intensive care units in hospitals with and without intermediate care units: A multicentre European cohort study. Crit Care 2014; 18:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bravata DM, Perkins AJ, Myers LJ, et al. : Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open 2021; 4:e2034266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matute-Villacís M, Moisés J, Embid C, et al. : Role of respiratory intermediate care units during the SARS-CoV-2 pandemic. BMC Pulm Med 2021; 21:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grosgurin O, Leidi A, Farhoumand PD, et al. : Role of intermediate care unit admission and noninvasive respiratory support during the COVID-19 pandemic: A retrospective cohort study. Respiration 2021; 100:786–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogrinc G, Davies L, Goodman D, et al. : SQUIRE 2.0 (Standards for Quality Improvement Reporting Excellence): Revised publication guidelines from a detailed consensus process. BMJ Qual Saf 2015; 25:986–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bapoje SR, Gaudiani JL, Narayanan V, et al. : Unplanned transfers to a medical intensive care unit: Causes and relationship to preventable errors in care. J Hosp Med 2011; 6:68–72 [DOI] [PubMed] [Google Scholar]
- 15.Escobar GJ, Greene JD, Gardner MN, et al. : Intra-hospital transfers to a higher level of care: Contribution to total hospital and intensive care unit mortality and length of stay. J Hosp Med 2011; 6:74–80 [DOI] [PubMed] [Google Scholar]
- 16.Robert R, Reignier J, Tournoux-Facon C, et al. ; Association des Réanimateurs du Centre Ouest Group: Refusal of intensive care unit admission due to a full unit: Impact on mortality. Am J Respir Crit Care Med 2012; 185:1081–1087 [DOI] [PubMed] [Google Scholar]
- 17.R Core Team: R: A Language and Environment for Statistical Computing. Vienna, Austria, R Foundation for Statistical Computing, 2021. Available at: https://www.R-project.org/. Accessed August 23, 2023 [Google Scholar]
- 18.Hager DN, Tanykonda V, Noorain Z, et al. : Hospital mortality prediction for intermediate care patients: Assessing the generalizability of the Intermediate Care Unit Severity Score (IMCUSS). J Crit Care 2018; 46:94–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simpson CE, Sahetya SK, Bradsher RW, et al. : Outcomes of emergency medical patients admitted to an intermediate care unit with detailed admission guidelines. Am J Crit Care 2017; 26:e1–e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hager DN, Chandrashekar P, Bradsher RW, et al. : Intermediate care to intensive care triage: A quality improvement project to reduce mortality. J Crit Care 2017; 42:282–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doctoroff L, Hsu DJ, Mukamal KJ: Trends in prolonged hospitalizations in the United States from 2001 to 2012: A longitudinal cohort study. Am J Med 2017; 130:483.e1–483.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao EJ, Yeluru A, Manjunath L, et al. : A long wait: Barriers to discharge for long length of stay patients. Postgrad Med J 2018; 94:546–550 [DOI] [PubMed] [Google Scholar]
- 23.Al-Rajhi A, Mardini L, Jayaraman D: The impact of implementation of an ICU consult service on hospital-wide outcomes and ICU-specific outcomes. J Intensive Care Med 2016; 31:478–484 [DOI] [PubMed] [Google Scholar]
- 24.Vincent JL: The continuum of critical care. Crit Care 2019; 23(Suppl 1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoo EJ, Damaghi N, Shakespeare WG, et al. : The effect of physician staffing model on patient outcomes in a medical progressive care unit. J Crit Care 2016; 32:68–72 [DOI] [PubMed] [Google Scholar]