Abstract
Osteosarcoma (OS) is the most common primary malignant bone tumor that more commonly occurs in children and adolescents. The most commonly used treatment for OS is surgery combined with chemotherapy, but the treatment outcomes are typically unsatisfactory. High rates of metastasis and post-treatment recurrence rates are major challenges in the treatment of OS. This underlines the need for studying the in-depth characterization of the pathogenetic mechanisms of OS and development of more effective therapeutic modalities. Previous studies have demonstrated the important role of the bone microenvironment and the regulation of signaling pathways in the occurrence and development of OS. In this review, we discussed the available evidence pertaining to the mechanisms of OS development and identified therapeutic targets for OS. We also summarized the available treatment modalities for OS and identified future priorities for therapeutics research.
Keywords: Osteosarcoma, Bone microenvironment, Signaling pathway
Introduction
Osteosarcoma (OS) is the most common primary malignant bone tumor, and it most commonly occurs in children and adolescents, followed by older adults aged more than 60 years.[1] OS is pathologically classified into the low-, intermediate-, or high-grades, and over 90% cases of OS have high-grade malignant tumors.[2] Approximately 25% patients with OS have metastatic disease, most commonly in the lungs.[3]
The currently available treatment modalities for OS include surgery, neoadjuvant chemotherapy, and postoperative chemotherapy, but the treatment outcomes are largely unsatisfactory.[4] OS is characterized by a high risk of metastasis and post-treatment recurrence.[5] The estimated 5-year survival rate of patients with recurrent or metastatic OS is less than 25%.[6] Furthermore, there has been no significant increase in the survival rate of patients with metastatic disease over the recent decades.[7] Therefore, tumor metastasis remains a primary concern in the treatment of OS.
Owing to the poor prognosis of OS, exploration of the molecular mechanism mediating the occurrence and development of OS is a key imperative to improve clinical treatment. The tumor microenvironment plays a key role in the proliferation, invasion, and metastasis of OS, and therapies targeting the tumor microenvironment can have a good effect.[8] Additionally, several studies have also demonstrated an essential role of dysregulation of genetic signaling pathways in the development of OS. Therefore, in this review, we focused on the role of the bone microenvironment and the signaling pathways in the context of OS. Additionally, we discussed the current clinical treatments for OS and the potential novel therapeutic strategies to improve the treatment of OS.
Regulation of the Bone Microenvironment in OS
Adipose tissue and OS development
In recent years, several studies have demonstrated a clear interplay between adipose tissue and carcinogenesis.[9] Adiponectin (adipocyte compliment related protein 30 [Acrp-30]), the most abundant adipose factor produced and secreted by adipose tissue, can act as a linking factor between adipose tissue and tumors. Studies found that Acrp-30 plays an anti-proliferative and pro-apoptotic role in cancer.[10,11] Therefore, Acrp-30-based therapy is a potential approach for cancer treatment. However, the unfavorable properties of Acrp-30, such as heavy molecular mass and reduced half-life/stability, hinder its usage in cancer.[12] The development of small and synthetic agonists has provided new potential therapeutic options. The first-generation small molecule lipocalin receptor agonist adiporone, the most widely studied non-peptide, is a potential candidate drug for lipocalin replacement therapy.[12] In the study by Sapio et al[13], adiporone was found to affect the cell cycle progression and inhibit proliferation of human OS cells, which was likely mediated via activation of the extracellular regulated protein kinase (ERK) and mammalian target of rapamycin (mTOR) signaling pathways. Although the initial preclinical results have highlighted the antiproliferative effects of the agonist in OS models, the underlying molecular mechanisms are not well characterized. Therefore, future research should not only focus on the screening of agonists but also elucidate its mechanism of action in OS to better target its application in the clinical setting.
Nerves and OS development
Emerging data suggest that many tumors are innervated by the nerves of the tumor microenvironment and that nerves are actively involved in the malignant progression of cancer.[14] The stimulatory effect of nerves seems to be related to the release of neurotransmitters (such as norepinephrine) from nerve endings in the tumor microenvironment, which activates the nerve signals of interstitial cells and cancer cells and promotes tumor progression.[15] The release of nerve growth factor (NGF) and other neurotrophic growth factors in tumor cells promotes the growth of tumor nerves, resulting in an increase in nerve density in the tumor microenvironment.[16] Nerve and neurotrophic growth factors are increasingly recognized as potential tumor biomarkers and therapeutic targets.[15] However, the role of tumor innervation in the context of OS has not been well investigated. Further studies to characterize the relationship between them can elucidate the pathogenesis, and help identify more effective targeted therapies for OS.
Acidity/hypoxia and OS development
Acidity and hypoxia are prevalent in the bone microenvironment and are associated with the development and progression of OS. Abnormal expression of some genes induced by acidity and hypoxia promotes the metastasis of OS [Table 1].
Table 1.
Hypoxia and acidosis in the bone microenvironment promote the metastasis of OS.
| Conditions | Genes | Interacting molecules | Efficacy | Reference |
|---|---|---|---|---|
| Hypoxia | MSTO2P | PD-L1 | Proliferation, invasion, and EMT↑ | [17] |
| Bcl-2 | MicroRNA-15a | Migration and invasion ↑ | [18] | |
| MicroRNA-33b | Proliferation and invasion↑ | [19] | ||
| IncRNA-FOXD2-AS1 | Proliferation and invasion↑ | [20] | ||
| PDGF/PDGFR | Proliferation and migration↑ | [21] | ||
| Acidity | CXCL1, CXCL2, CXCL4,CXCL5, IL-6, IL-8 | – | Growth and metastasis↑ | [22] |
EMT: Epithelial-to-mesenchymal-transition; OS: Osteosarcoma; PD-L1: Programmed cell death-ligand 1; PDGF: Platelet-derived growth factor; PDGFR: PDGF receptor.
The Regulatory Role of Signaling Pathways in OS
Wnt/β-catenin signaling pathway
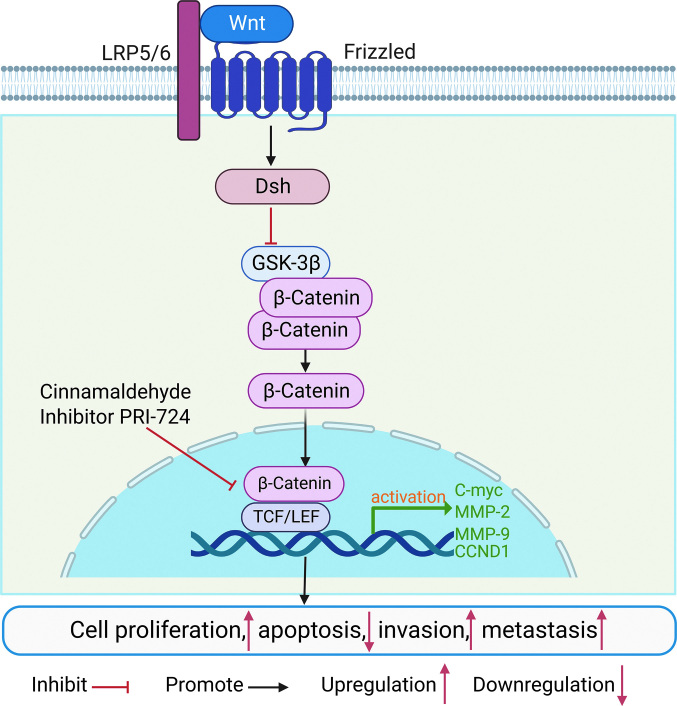
The typical Wnt signaling pathway is the Wnt/β-catenin signaling pathway [Figure 1]. Several studies have shown that abnormal activation of Wnt signaling and high expression of β-catenin are associated with abnormal histomorphology and abnormal cell proliferation and differentiation in OS, ultimately leading to the development of OS. Accumulation of β-catenin in the cytoplasm to a certain concentration induces its translocation to the nucleus, where it binds to the nuclear transcription factor T-cell factor/lymphoid enhancing factor (TCF/LEF), leading to the activation and expression of downstream target gene promoters, thereby promoting tumor formation.[23] Glycogen synthase kinase-3β (GSK-3β) in the cytoplasm plays an important role in the degradation of β-catenin and can inhibit the Wnt signaling pathway by reducing the stability of β-catenin.[24] In a study by Huang et al[25], cinnamaldehyde was shown to inhibit the classical Wnt/β-catenin signaling by down-regulating β-catenin and inducing phosphorylation of downstream target proteins C-myc and matrix metallopeptidase 7 (MMP-7). Additionally, cinnamaldehyde showed a significant inhibitory effect on the phosphatidylinositol 3 kinase/protein kinase B (PI3K/AKT) pathway, which regulates tumor cell proliferation and survival. Activated AKT and GSK-3β can induce the expression of a variety of anti-apoptotic protein genes or inhibit apoptosis through a variety of signaling-coupled pathways. GSK-3β is a branch downstream of AKT and its activity enhances the phosphorylation of GSK-3β, leading to a decrease in its activity.[26,27] Cinnamaldehyde may inhibit the accumulation of β-catenin and p-AKT by enhancing GSK-3β activity, thereby suppressing the growth of OS.[25] A number of small molecule compounds also play a key role in OS by regulating the Wnt/β-catenin signaling pathway. Fang et al[28] showed that the small-molecule Wnt/β-catenin inhibitor PRI-724 reduces the proliferation, invasion, and metastasis of OS by suppressing Wnt/β-catenin-mediated transcription. In the research of Tan et al[29], dihydrotanshinone I was found to inhibit the growth of OS via the Wnt/β-catenin signaling pathway. The above findings indicate that the Wnt/β-catenin pathway is strongly associated with OS invasion and metastasis.
Figure 1.
Wnt/β-catenin signaling pathway and OS. Wnt ligands bind to the Frizzled and low density LRP5/6. Thereafter, this signal activates the protein dishevelled (Dsh) in the cytoplasm. Dsh can reduce the degradation of β-catenin by inhibiting the activity of GSK-3β kinase. Accumulation of higher amounts of β-catenin induces its translocation from the cell membrane to the nucleus, where it acts as a transcriptional co-activator of transcription factors to participate in the TCF/LEF family. C-myc: Cellular-myelocytomatosis viral oncogene; CCND1:CyclinD1; Dsh: Dishevelled; GSK-3β: Glycogen synthase kinase-3 beta; LRP5/6: Lipoprotein receptor-related protein5 and 6; MMP-2: Matrix Metallo-peptidase 2; OS: Osteosarcoma; TCF: T-cell factor; LEF: Lymphoid enhancer-binding factor; Wnt: Wingless/Integrated.
PI3K/AKT signaling pathway
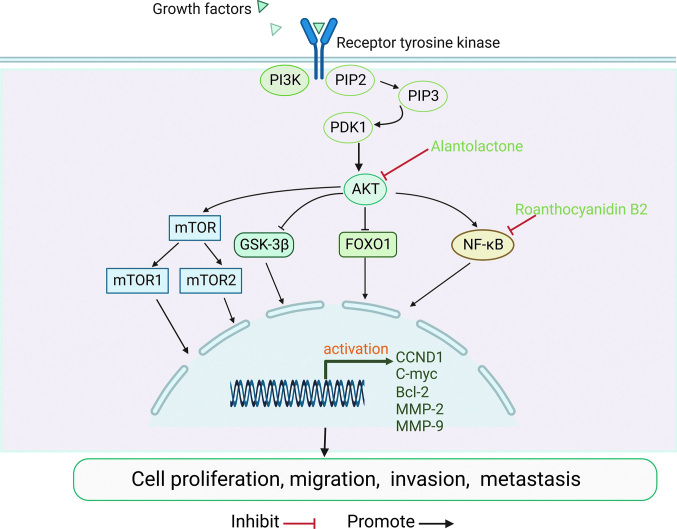
Several studies have demonstrated abnormal expression of components of the PI3K/AKT signaling pathway in human cancers, including OS. Altered expression levels and activity of proteins involved in the PI3K/AKT pathway are most commonly observed in OS. Dysregulated PI3K/AKT pathway was shown to promote the proliferation and metastasis of OS cells[30] [Figure 2]. Human epidermal growth factor receptor 4 (HER4), a member of the human epidermal growth factor (EGF) receptor family, is strongly associated with poor prognosis in patients with OS.[31] Studies have found that HER4 may promote the growth and metastasis of OS via the PI3K/AKT pathway.[32] The HER4/PI3K/AKT pathway is a potential target for OS. Transforming growth factor-β (TGF-β) is a multifunctional cytokine that plays an important role in cell proliferation and differentiation. TGF-β is highly expressed in a variety of tumors and promotes tumor invasion and metastasis. TGF-β was shown to be associated with poor prognosis and to promote the progression of OS via activation of the PI3K/AKT pathway.[31] A key downstream target of the PI3K/AKT pathway is mTOR, which is required to promote cell proliferation, migration, and survival. Additionally, other important targets affected by AKT include GSK 3β and nuclear factor-κB (NF-κB).[33] These important findings can help inform the development of inhibitors for the corresponding target molecules. In recent years, anti-cancer properties of natural compounds have become a research hotspot. In a study by Wu et al[34],proanthocyanidin B2 was shown to inhibit the proliferation and induce apoptosis of OS cells by downregulating the PI3K/AKT/NF-κB signaling pathway. Zhang et al[35] discovered that alantolactone (ALT) inhibits the growth of OS cells by downregulating the PI3K/AKT signaling pathway, suggesting that ALT is a potential candidate for the treatment of OS. In summary, the search for effective targets in the PI3K/AKT signaling pathway may help clarify the mechanism of OS metastasis and facilitate the development of treatment strategies.
Figure 2.
PI3K/AKT signaling pathway and OS. After the ligand binds to the receptor, PI3K binds to PIP2 on the inner membrane inducing phosphorylation of PIP2 to PIP3. Thereafter, PIP3 activates AKT through PDK1. AKT can activate the proteins mTOR and NK-κB or inhibit GSK-3β and FOXO1, and then activate the expression of downstream target genes. AKT: Protein Kinase B; Bcl-2:B-cell lymphoma-2; C-myc:Cellular-myelocytomatosis viral oncogene; CCND1: CyclinD1; FOXO1: Forkhead box O1; GSK-3β: Glycogen synthase kinase-3β; NK-κB: Nuclear factor-κB; MMP-2: Marix Metallo-peptidase 2; mTOR: Mammalian target of rapamycin; OS: Osteosarcoma; PDK1: Pyruvate dehydrogenase kinase, isozyme1; PI3K/AKT: Phosphatidylinositol 3 kinase/protein kinase B; PIP2: Phosphatidylinosital biphosphate; PIP3: Phosphatidylinositol triphosphate; RTK: Receptor tyrosine kinase.
Hedgehog signaling pathway
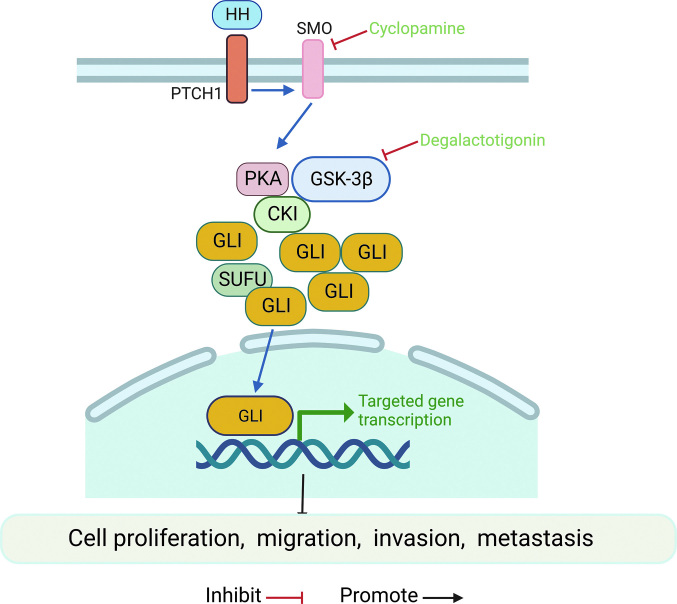
The hedgehog (HH) pathway mainly participates in cell growth, differentiation, and tissue polarity,[36] and its aberrant activation also leads to tumorigenesis and supports an aggressive tumor phenotype.[37] Relevant studies have demonstrated a close relationship of the HH signaling pathway with tumorigenesis and metastasis of OS.[38] Activation of the HH pathway mediated by multiple genes significantly promotes OS invasion and metastasis [Figure 3]. Yao et al[36] reported the role of the gene encoding ribosomal protein S3 (RPS3) in regulating HH/glioma-associated oncogene homolog-2 (GLI-2) signaling in OS invasion and metastasis. GLI-2 has been reported to promote malignant behaviors such as the proliferation, migration, and invasion of MSCs and OS cells.[39] RPS3 is a target gene of GLI-2. Knockdown of GLI-2 significantly reduces RPS3 expression, while GLI-2 overexpression upregulates RPS3 expression.[40] Additionally, the HH signaling pathway was found to play an inhibitory role in the development of OS by regulating the Wnt/β-catenin signaling pathway. Loss of recombinant patched1 (Ptch1) up-regulates the expression of Wnt5a/6 and enhances the activation of β-catenin. Inhibition of the Wnt/β-catenin pathway inhibited the development of bone abnormalities, including OS, suggesting that the Wnt/β-catenin pathway could be a pharmacological target for OS.[41] Recent research and application of small molecule compounds suggest their potential role in the treatment of OS. For example, degalactotigonin was shown to inhibit the growth and metastasis of OS via modulation of GSK-3β inactivation-mediated repression of the HH pathway. Additionally, cyclopamine was also found to inhibit OS development.[42,43] The above findings indicate an important role of the HH signaling pathway in the occurrence of OS. However, the activated HH pathway is not the main driver of malignancy. Further research on combination therapy along with inhibitors of other signaling pathways may provide an effective treatment approach for OS.
Figure 3.
Hedgehog signaling pathway and OS. The HH ligand binds to the Ptch1 receptor. SMO attenuates and inhibits the proteolysis of GLI proteins. Thereafter, the active GLI protein translocates to the nucleus and activates the downstream transcription factors. GLI: Glioma-associated oncogene homolog; GSK-3β: Glycogen synthase kinase-3β; HH: Hedgehog; OS: Osteosarcoma; PTCH1: Patched homolog 1; PKA: Protein Kinase A; SUFU: Suppressor of fused homolog; SMO: Smoothened.
Notch signaling pathway
The Notch signaling family consists of four receptors and five Delta/Serrate/Lag2 (DSL) ligands, called Notch-1 to Notch-4, as well as Jaggeg-1 and Jaggeg-2 (Jag-1 and Jag-2) and Delta-like-1, Delta-3, and Delta-4 (DLL-1, DLL-3, and DLL-4). The ligands interact with the receptors to activate the channels, which in turn play an important role[44] [Figure 4]. In recent years, an increasing body of evidence has implicated aberrant Notch activation in tumor process, including cell metastasis.[45] In particular, activation of the Notch signaling pathway is closely associated with the migration and metastasis of OS.[46] Therefore, Notch signaling has emerged as one of the important potential targets for the development of new therapeutic strategies. The E3 ubiquitin ligase Deltex-1 (DTX1) is a key negative regulator of Notch signaling, and DTX1 silencing leads to an increase in the expression of Notch1 in OS cells. Furthermore, activation of putative phosphatidylinositol 5-phosphate 4-kinaseγ (PI5P4Kγ) inhibits Notch activation and recirculation rates.[47] These findings suggest that inhibition of Notch signaling at different sites may be a novel therapeutic strategy to prevent OS invasion and metastasis. Long non-coding RNAs (IncRNAs) and mRNA-interfering complementary RNAs (microRNAs) play an important role in the pathogenesis of human diseases. Numerous studies have documented the roles of lncRNAs and microRNAs in the occurrence and development of OS by regulating the Notch signaling pathway. Chen et al[48] discovered that the lncRNA MEG3 inhibits OS cell proliferation and promotes apoptosis by regulating the Notch signaling pathway. Moreover, miR-135B has been shown to promote OS recurrence and pulmonary metastasis through the targeted activation of the Notch signaling and Wnt/β-catenin pathways.[49] Additional studies have demonstrated an association with Wnt signaling in the regulation of Notch signaling in OS. Wnt10b, a classic Wnt signaling molecule known to have important effects on bone, upregulates genes associated with Notch signaling (particularly Notch-1), while Notch repressors significantly downregulated, leading to the activation of classical Notch-responsive genes including hes-1 and hey-1. In the U2OS OS cell model, Wnt10b was shown to significantly regulate the Wnt and Notch pathways.[50]
Figure 4.
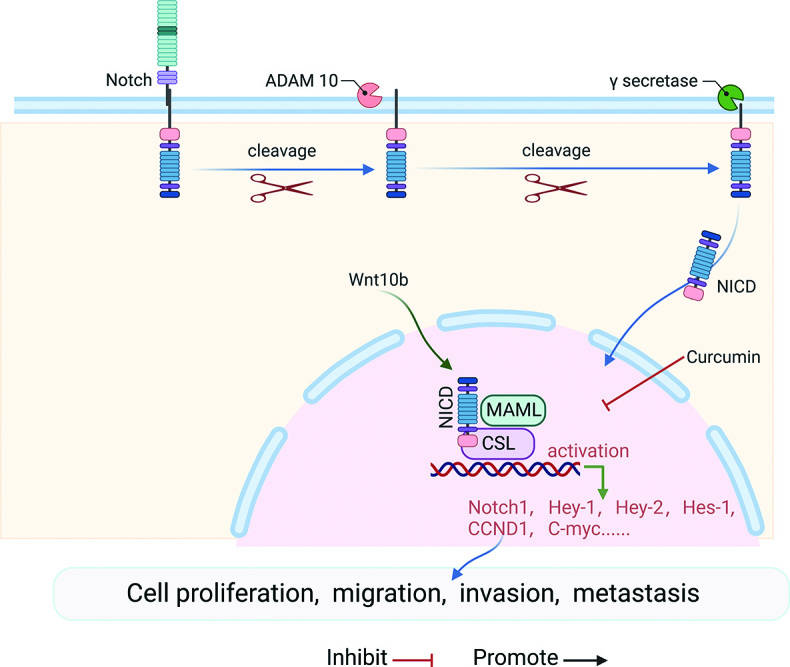
Notch signaling pathway and OS. After ligand binding to the receptor, the complex is cleaved by ADAM and γ-secretase yielding the NICD, and NICD is translocated into the nucleus. It binds to the transcription factor cbf-1, CSL and transcriptional coactivator of the MAML. This complex activates transcription of the target gene. ADAM: A cellular disintegrin and metalloproteinase; CCND1: CyclinD1; C-myc: Cellular-myelocytomatosis viral oncogene; CSL: Suppressor of hairless and lag; Hey-1: Hes Related Family BHLH Transcription Factor With YRPW Motif 1; Hes-1:Hes family bHLH transcription factor 1; MAML: Mastermind-like family members; NICD: Notch intracellular domain; Notch1: Notch homolog 1; OS: Osteosarcoma.
MAPK/ERK signaling pathway
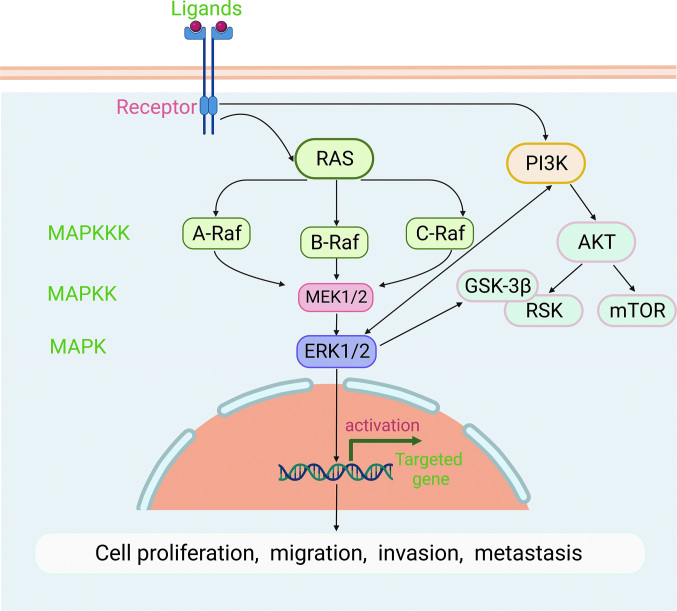
The typical mitogen-activated protein kinase/extracellular regulated protein kinase (MAPK/ERK) pathway consists of three types of mitogen-activated protein kinase kinase (MAPKK), namely A-cellular rapidly accelerated fibrosarcoma (A-RAF), B-cellular rapidly accelerated fibrosarcoma (B-RAF), and C-rapidly accelerated fibrosarcoma (C-RAF) kinase. The next level is MAPKK including recombinant human mitogen activated kinase kinase 1 (MEK1) and recombinant human mitogen activated kinase kinase 2 (MEK2). Finally, further downstream are extracellular regulated protein kinase1 (ERK1) and extracellular regulated protein kinase2 (ERK2), which activate the expression of downstream target genes[51] [Figure 5]. Abnormal activation of this pathway is closely related to cell transformation and carcinogenesis.[52] Dual-specificity protein phosphatase1 (DUSP1), a phosphatase, dephosphorylates MAPKs. DUSP1 expression is significantly increased in patients with metastatic disease and patients who received chemotherapy, suggesting that the DUSP1 gene may be associated with OS metastasis and drug resistance. Additionally, overexpression of DUSP1 was shown to be strongly associated with a poor prognosis in patients with OS.[53] Miao et al[54] also found that galectin-1 knockdown inhibits the growth and invasion of OS cells by inhibiting the MAPK/ERK pathway. These data suggest that DUSP1/galectin-1 may be a potential target for the treatment of OS. A complex regulatory network is involved in the occurrence of OS, and in most cases, it is regulated by multiple pathways. Studies have demonstrated an interaction between the MAPK/ERK and PI3K/AKT pathways, and the active targets include PI3K and GSK-3β.[55] These findings suggest that screening for target inhibitors that act on both MAPK/ERK and PI3K/AKT pathways can help inhibit the development of OS more effectively. In general, the activation of the MAPK/ERK signaling pathway has a closer relationship with the malignant progression of OS, and thus the identification of effective therapeutic targets for OS treatment is extremely important.
Figure 5.
MAPK/ERK signaling pathway and OS. After acting on and RAS, A-RAF, B-RAF, and C-RAF are activated and phosphorylate its dual-specific protein kinase substrates MEK1 and MEK2. Thereafter, MEK1/2 phosphorylates its substrate ERK1/2. The MAPK/ERK signaling pathway interacts with PI3K/AKT, and its targets include PI3K, GSK-3β, and RAS. AKT: Protein Kinase B; A-RAF: A-cellular rapidly accelerated fibrosarcoma; C-RAF: C-rapidly accelerated fibrosarcoma; ERK1/2: Extracellular regulated protein kinases1/2; GSK-3β: Glycogen synthase kinase-3β; MEK1: Mitogen activated kinase kinase 1; MAPK/ERK: Mitogen-activated protein kinase/extracellular regulated protein kinase; MAPKK: Mitogen-activated protein kinase kinase; MAPKKK: Mitogen-activated protein kinase kinase kinase; mTOR: Mammalian target of rapamycin; OS: Osteosarcoma; PI3K: Phosphoinositide 3-Kinase; RSK: Ribosomal S6 Kinase; RAS: Rapidly accelerated sarcoma.
Novel Therapies for OS
Immunotherapy
Since the 1970s, immunotherapy has been widely used in patients with OS, and some curative effects have been achieved.[56] Currently available immunotherapeutic modalities mainly include the application of immune checkpoint monoclonal antibody, cytokines, and dendritic cells (DCs).
The emergence of anti-immune checkpoint monoclonal antibodies represents a breakthrough in cancer immunotherapy.[56] Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programed death receptor-1 (PD-1) are the main inhibitory receptors expressed on T cells and are considered novel therapeutic targets in the context of various advanced malignant tumors.[57] Programmed cell death ligand 1 (PD-L1) is the ligand of PD-1. In patients with OS, the expression levels of both PD-L1 and PD-1 are negatively correlated with prognosis.[58] Merchant et al[59] found that when pediatric patients with relapsed or refractory OS were pretreated with the CTLA-4 antibody ipilimumab, approximately one-quarter developed stable disease, suggesting moderate activity. Among adult patients with OS treated with the anti-PD-1 antibody nivolumab, one-third showed partial response, and the others had stable disease.[60] In a successfully constructed mouse model of advanced OS, the combined application of anti-CTLA-4 and anti-PD-L1 antibodies improved the overall survival rate, while anti-CTLA-4 antibody treatment alone showed no obvious effect.[61] These findings suggest that immune checkpoint therapy may be effective in some patients with OS, but it is likely to require a combination of corresponding antibody therapies. Additionally, immune checkpoints are the targets of OS treatment, and further studies are required to determine the effectiveness of immune checkpoint inhibitors.
Cytokines have been used to treat malignant tumors. Interferon-α (IFN-α) is currently the most extensively used therapy for various tumors including melanoma, hepatocellular carcinoma, and renal carcinoma.[62] IFN-α was also found to have an inhibitory effect on the growth of human OS cells. In a study by Zhao et al[63], IFN-α was found to inhibit the invasion of human OS MG-63 cells and enhance cisplatin-mediated apoptosis and autophagy. Moreover, the combination of chemotherapy and IFN-α may be a novel treatment method for OS, which needs to be investigated in further experimental research. Additionally, studies have also demonstrated the antiproliferative and proapoptotic effects of recombinant interferon-α-2b (IFN-α-2b) in OS models.[64] In a randomized clinical trial, patients with OS were treated with methotrexate, adriamycin and cisplatin (MAP) with or without IFN-α-2b. The 5-year overall survival of patients treated with MAP+IFN-α-2b was higher than that of those treated with MAP alone, further confirming the antitumor effects of IFNα-2b.[65]
Dendritic cells (DCs) are antigen-presenting cells that play a key role in the initiation and regulation of innate and adaptive immune responses and can activate both CD4 and CD8 T cells.[66] He et al[67] found that stimulating DCs with tumor lysates significantly increases the activity of cytotoxic T lymphocytes (CTLs), thereby increasing serum IFN levels. Studies have reported the effect of tumor-lysed DCs and anti-CTLA-4 antibodies on the treatment of OS models. Mice that received tumor-lysed DCs and anti-CTLA-4 antibodies had decreased number of regulatory T lymphocytes and increased number of CD8+ T lymphocytes, and showed inhibition of metastatic growth, prolonged lifespan, decreased number of splenic regulatory T lymphocytes, and increased serum interferon-gamma level.[68] Additionally, the combined application of anti-glucocorticoid-induced tumor necrosis factor receptor (anti-gitr) antibodies and tumor-lysing DCs was found to enhance the systemic immune response.[69] Over the years, no serious adverse effects have occurred in clinical studies of DC-based immunotherapy.
Targeted therapy
Compared with traditional therapies, targeted therapy is a highly selective and low-toxic treatment method for cancer. Currently, molecular targeted therapies have shown remarkable efficacy in the treatment of various cancers, such as lung, colorectal, and ovarian cancers.[70]
Although the epidermal growth factor receptor (EGFR) is normally involved in tissue development and maintenance, it can promote tumor growth, invasion, and metastasis by activating PIK-3/AKT, phospholipase C-protein kinase C (PLC-PKC), and signal transducers and activators of transcription (STAT) signaling pathways. Additionally, considering the limitations of conventional chemotherapy, the EGFR is currently considered an important target.[71] Studies have found that the expression of the EGFR is associated with an improved prognosis in a dose-responsive manner.[72] Wang et al[72] observed increased levels of phosphorylated EGFR levels in OS cells treated with gemcitabine. Compared with gemcitabine alone, gemcitabine combined with EGFR knockdown induced G1 phase cell cycle arrest in OS cells, which exerted a stronger inhibitory effect on the proliferation of OS cells. Conversely, EGFR overexpression counteracted the growth-inhibitory and proapoptotic effects of gemcitabine on OS cells.
Vascular endothelial growth factor (VEGF) promotes endothelial cell migration, proliferation, and angiogenesis.[73] Studies have shown VEGF expression is a validated prognostic biomarker for OS.[74] VEGF and its receptors are the key mediators of tumor angiogenesis, and several VEGF-targeted drugs have been developed.[75] Regorafenib is an oral multikinase inhibitor that affects the vascular and tumor microenvironment by targeting specific kinase proteins such as vascular endothelial growth factor receptor 1,2,3 (VEGFR 1, 2, and 3), platelet-derived growth factor receptor beta (PDGFRb) and rapidly accelerated fibrosarcoma (RAF).[75] The NCCN guidelines recommend regorafenib as the first-choice treatment for patients with relapsed or metastatic OS.[76] Cabozantinib, a multikinase inhibitor that mainly targets VEGFR 2, exerts its anticancer effects primarily by inhibiting tumor receptor tyrosine kinases, and its antitumor activity has been confirmed both in vivo and in vitro.[76,77] Sorafenib is also an oral agent that targets VEGFR 1, 2, and 3.[76] In patients with recurrent or unresectable OS, sorafenib monotherapy alone was found only to temporarily inhibit the progression of OS, with little survival benefit, indicating the need for more effective combination therapy to achieve permanent remission.[75] The lack of efficacy of sorafenib monotherapy is due to its action on the mTOR pathway. Therefore, combining sorafenib with an mTOR inhibitor may improve its efficacy.[75] Additionally, a number of studies have shown that sorafenib can also be used in combination with other drugs such as cisplatin and gemcitabine to exert an inhibitory effect in OS.[76,78]
Poly adp-ribose polymerase (PARP), cyclin-dependent kinases 4 and 6 (CDK4/6), and PI3K inhibitors may also play a certain role in the targeted treatment of OS. Administration of the poly adp-ribose polymerase 1 (PARP1) inhibitor olaparib was shown to induce cell death in OS cell lines U2-OS, Saos-2, and MG-63.[79] The CDK4/CDK6 inhibitor palbociclib may be a new targeted therapy for patients with OS; however, more research is required to investigate the efficacy of palbociclib in patients with OS and to identify its side effects.[80] Additionally, application of PI3K inhibitors has also achieved certain results in the treatment of OS. In the study by Chen et al[81], PI3K inhibitors were found to reverse anlotinib resistance in OS, inhibiting OS cell development in combination with anlotinib. These findings provide the rationale for further studies of the clinical application of PI3K inhibitors in the treatment of anlotinib-refractory OS.
Research on the use of exosomes as a medium for targeted therapy has become a contemporary research hot spot. In OS, exosomes can be used both as diagnostic markers and as potential therapeutics due to their effects on tumor initiation and progression target.[82] Liu et al[83] demonstrated that exosome-loaded miR-769-5p can modulate OS progression by targeting DUSP1. Therefore, artificially overexpressing or inhibiting some miRNAs and using exosomes as carriers to target the proliferation or invasion of OS cells may be a potential approach to prevent OS progression. Currently, miRNA-loaded exosomes have shown great potential for the treatment of OS. However, there are several challenges in the application of exosomes as OS-targeted therapeutic carriers that need to be solved.[82]
Gene therapy
Gene mutation is the most fundamental cause of OS. Therefore, gene research plays an important role in its treatment. OS gene therapy methods mainly focus on tumor suppressor genes, suicide genes, combining genes, and immune genes.[84] Tumor suppressor genes mainly include P53, P21, and Rb; among these, P53 is the most well characterized in the literature. Studies have demonstrated that OS patients often have P53 mutations.[85] Wu et al[86] found that P53 protein expression can be used as a prognostic biomarker to predict overall survival of OS patients, which further positions P53 as an entry point for OS gene therapy. Some studies have demonstrated that combining gene therapy with other treatments may not only have synergistic effects but also reduce the adverse reactions caused by drug therapy alone. Combining gene therapy with other treatments for OS patients is expected to gain recognition in future as a meaningful approach to gene therapy, especially transgenic T-cell therapy. However, since gene therapy is still in the experimental stage, it has not yet been put into clinical use.[84]
Conclusions and Perspectives
This article reviewed the relationship of OS and the bone microenvironment. We also summarized the role of several typical signaling pathways in promoting the malignant progression of OS to further improve our understanding of OS pathogenesis [Figure 6]. Surgical resection, neoadjuvant chemotherapy, and postoperative chemotherapy are the main treatment modalities for OS, but none have achieved satisfactory outcomes. Tumor drug resistance is a key contributor to unsatisfactory outcomes. Studies have found that the acidic tumor microenvironment of OS can lead to multidrug resistance. Can the acidity of the tumor microenvironment be reduced to prevent multidrug resistance of OS? Additionally, the reasons for the poor efficacy of OS treatment also include the relative rarity and heterogeneity of this cancer and the lack of understanding of tumor-specific antigens. Multi-molecular targeted therapy combined with other treatment methods seems to have made some progress in the treatment of OS, but there are still some problems. For example, not only might multi-target drugs increase toxicity but also it is difficult to identify which specific kinase inhibition will produce anti-tumor effects.[75]Therefore, the issue of selecting appropriate drugs for combination therapy remains to be resolved. Additionally, the application of small molecule natural compounds has been a hot topic in recent years, but at the same time there are some limitations. For example, curcumin, a natural polyphenol, can inhibit the proliferation of OS cell lines. However, the clinical application of curcumin is greatly limited due to its innate properties such as hydrophobicity and low bioavailability, which affect its anticancer effects. To improve its therapeutic effect, it may potentially be used in combination with immunotherapy, chemotherapy, and other approaches.
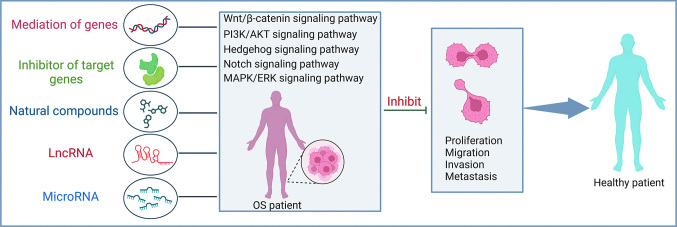
Figure 6.
Major OS-related signaling pathway. Mediation of genes, inhibitor of target genes, natural compounds, IncRNA and microRNA can participate in the regulation of OS-related signaling pathways including Wnt/β-catenin, PI3K/AKT, HH, Notch and MAPK/ERK signaling pathways, thereby inhibiting the malignant behavior of OS. HH: Hedgehog; LncRNA: Long non-coding RNA; MAPK/ERK: Mitogen-activated protein kinase/extracellular regulated protein kinase; OS:Osteosarcoma; PI3K/AKT: Phosphatidylinositol 3 kinase/protein kinase B.
In general, the mechanisms of OS development are complex and involve multiple biomolecular and genetic signaling pathways. Therefore, further research is required to clarify the mechanisms to provide precise treatments and improve the prognosis of patients with OS.
Funding
This study was supported by a grant from the National Natural Science Foundation of China (No.81871814).
Conflicts of interest
None.
Footnotes
Jichao Bian and Yang Liu contributed equally to this study.
How to cite this article: Bian JC, Liu Y, Zhao XW, Meng CY, Zhang YM, Wang GD. Research progress in the mechanism and treatment of osteosarcoma. Chin Med J 2023;136:2412–2420. doi: 10.1097/CM9.0000000000002800
References
- 1.Lv Y Wu L Jian H Zhang C Lou Y Kang Y, et al. . Identification and characterization of aging/senescence-induced genes in osteosarcoma and predicting clinical prognosis. Front Immunol 2022; 13: 997765. doi: 10.3389/fimmu.2022.997765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kager L, Tamamyan G, Bielack S. Novel insights and therapeutic interventions for pediatric osteosarcoma. Future Oncol 2017; 13: 357–368. doi: 10.2217/fon-2016-0261. [DOI] [PubMed] [Google Scholar]
- 3.Nomura M Rainusso N Lee YC Dawson B Coarfa C Han R, et al. . Tegavivint and the β-catenin/ALDH axis in chemotherapy-resistant and metastatic osteosarcoma. J Natl Cancer Inst 2019;111: 1216–1227. doi: 10.1093/jnci/djz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Q, Liu J, Wu B, Wang X, Jiang Y, Zhu D. Role of extracellular vesicles in osteosarcoma. Int J Med Sci 2022;19: 1216–26. doi: 10.7150/ijms.74137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cersosimo F Lonardi S Bernardini G Telfer B Mandelli GE Santucci A, et al. . Tumor-associated macrophages in osteosarcoma: From mechanisms to therapy. Int J Mol Sci 2020;21: 5207. doi: 10.3390/ijms21155207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li HB Huang G Tu J Lv DM Jin QL Chen JK, et al. . METTL14-mediated epitranscriptome modification of MN1 mRNA promote tumorigenicity and all-trans-retinoic acid resistance in osteosarcoma. EBioMedicine 2022;82: 104142. doi: 10.1016/j.ebiom.2022.104142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ning B, Liu Y, Huang T, Wei Y. Autophagy and its role in osteosarcoma. Cancer Med 2023;12: 5676–87. doi: 10.1002/cam4.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binnewies M Roberts EW Kersten K Chan V Fearon DF Merad M, et al. . Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 2018;24: 541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nigro E, Daniele A, Salzillo A, Ragone A, Naviglio S, Sapio L. AdipoRon and other adiponectin receptor agonists as potential candidates in cancer treatments. Int J Mol Sci 2021;22: 5569. doi: 10.3390/ijms22115569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nigro E Orlandella FM Polito R Mariniello RM Monaco ML Mallardo M, et al. . Adiponectin and leptin exert antagonizing effects on proliferation and motility of papillary thyroid cancer cell lines. J Physiol Biochem 2021;77: 237–248. doi: 10.1007/s13105-021-00789-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Zazzo E Polito R Bartollino S Nigro E Porcile C Bianco A, et al. . Adiponectin as link factor between adipose tissue and cancer. Int J Mol Sci 2019;20: 839. doi: 10.3390/ijms20040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otvos L, Jr. Potential adiponectin receptor response modifier therapeutics. Front Endocrinol (Lausanne) 2019;10: 539. doi: 10.3389/fendo.2019.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sapio L Nigro E Ragone A Salzillo A Illiano M Spina A, et al. . AdipoRon affects cell cycle progression and inhibits proliferation in human osteosarcoma cells. J Oncol 2020;2020: 7262479. doi: 10.1155/2020/7262479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boilly B, Faulkner S, Jobling P, Hondermarck H. Nerve dependence: From regeneration to cancer. Cancer Cell 2017;31: 342–354. doi: 10.1016/j.ccell.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Rowe CW Dill T Griffin N Jobling P Faulkner S Paul JW, et al. . Innervation of papillary thyroid cancer and its association with extra-thyroidal invasion. Sci Rep 2020;10: 1539. doi: 10.1038/s41598-020-58425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayakawa Y Sakitani K Konishi M Asfaha S Niikura R Tomita H, et al. . Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell 2017;31: 21–34. doi: 10.1016/j.ccell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi C, Huang CM, Wang B, Sun TF, Zhu AX, Zhu YC. Pseudogene MSTO2P enhances hypoxia-induced osteosarcoma malignancy by upregulating PD-L1. Biochem Biophys Res Commun 2020;530: 673–679. doi: 10.1016/j.bbrc.2020.07.113. [DOI] [PubMed] [Google Scholar]
- 18.Leng J, Song Q, Zhao Y, Wang Z. miR-15a represses cancer cell migration and invasion under conditions of hypoxia by targeting and downregulating Bcl-2 expression in human osteosarcoma cells. Int J Oncol 2018;52: 1095–1104. doi: 10.3892/ijo.2018.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y, Yang C, Wang K, Liu X, Liu Q. MicroRNA-33b inhibits the proliferation and migration of osteosarcoma cells via targeting hypoxia-inducible factor-1α. Oncol Res 2017;25: 397–405. doi: 10.3727/096504016X1474333753446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren Z, Hu Y, Li G, Kang Y, Liu Y, Zhao H. HIF-1α induced long non-coding RNA FOXD2-AS1 promotes the osteosarcoma through repressing p21. Biomed Pharmacother 2019;117: 109104. doi: 10.1016/j.biopha.2019.109104. [DOI] [PubMed] [Google Scholar]
- 21.Zhang D Cui G Sun C Lei L Lei L Williamson RA, et al. . Hypoxia promotes osteosarcoma cell proliferation and migration through enhancing platelet-derived growth factor-BB/platelet-derived growth factor receptor-β axis. Biochem Biophys Res Commun 2019;512: 360–366. doi: 10.1016/j.bbrc.2019.03.040. [DOI] [PubMed] [Google Scholar]
- 22.Avnet S Di Pompo G Chano T Errani C Ibrahim-Hashim A Gillies RJ, et al. . Cancer-associated mesenchymal stroma fosters the stemness of osteosarcoma cells in response to intratumoral acidosis via NF-κB activation. Int J Cancer 2017;140: 1331–1345. doi: 10.1002/ijc.30540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sha L, Ma D, Chen C. Exosome-mediated Hic-5 regulates proliferation and apoptosis of osteosarcoma via Wnt/β-catenin signal pathway. Aging (Albany NY) 2020;12: 23598–23608. doi: 10.18632/aging.103546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang J Li Y Wang X Hu S Wang H Shi Q, et al. . Polyphyllin I suppresses human osteosarcoma growth by inactivation of Wnt/β-catenin pathway in vitro and in vivo. Sci Rep 2017;7: 7605. doi: 10.1038/s41598-017-07194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Y Chen J Yang S Tan T Wang N Wang Y, et al. . Cinnamaldehyde inhibits the function of osteosarcoma by suppressing the Wnt/β-catenin and PI3K/Akt signaling pathways. Drug Des Devel Ther 2020;14: 4625–4637. doi: 10.2147/DDDT.S277160. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Zhang Y, Cheng H, Li W, Wu H, Yang Y. Highly-expressed P2 × 7 receptor promotes growth and metastasis of human HOS/MNNG osteosarcoma cells via PI3K/Akt/GSK3β/β-catenin and mTOR/HIF1α/VEGF signaling. Int J Cancer 2019;145: 1068–1082. doi: 10.1002/ijc.32207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang GH Liu N He MT Yang J Liang ZJ Gao XJ, et al. . Transcriptional regulation of Runx2 by HSP90 controls osteosarcoma apoptosis via the AKT/GSK-3β/β-catenin signaling. J Cell Biochem 2018;119: 948–959. doi: 10.1002/jcb.26260. [DOI] [PubMed] [Google Scholar]
- 28.Fang F VanCleave A Helmuth R Torres H Rickel K Wollenzien H, et al. . Targeting the Wnt/β-catenin pathway in human osteosarcoma cells. Oncotarget 2018;9: 36780–36792. doi: 10.18632/oncotarget.26377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan T Chen J Hu Y Wang N Chen Y Yu T, et al. . Dihydrotanshinone I inhibits the growth of osteosarcoma through the Wnt/β-catenin signaling pathway. Onco Targets Ther 2019;12: 5111–5122. doi: 10.2147/OTT.S204574. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Chen H Pan R Li H Zhang W Ren C Lu Q, et al. . CHRDL2 promotes osteosarcoma cell proliferation and metastasis through the BMP-9/PI3K/AKT pathway. Cell Biol Int 2021;45: 623–632. doi: 10.1002/cbin.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang SL Zhong GX Wang XW Yu FQ Weng DF Wang XX, et al. . Prognostic significance of the expression of HER family members in primary osteosarcoma. Oncol Lett 2018;16: 2185–2194. doi: 10.3892/ol.2018.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Huang Q, Wang S, Huang Z, Yu F, Lin J. HER4 promotes the growth and metastasis of osteosarcoma via the PI3K/AKT pathway. Acta Biochim Biophys Sin (Shanghai) 2020;52: 345–362. doi: 10.1093/abbs/gmaa004. [DOI] [PubMed] [Google Scholar]
- 33.Angulo P Kaushik G Subramaniam D Dandawate P Neville K Chastain K, et al. . Natural compounds targeting major cell signaling pathways: A novel paradigm for osteosarcoma therapy. J Hematol Oncol 2017;10: 10. doi: 10.1186/s13045-016-0373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu X Yu H Zhou H Li Z Huang H Xiao F, et al. . Proanthocyanidin B2 inhibits proliferation and induces apoptosis of osteosarcoma cells by suppressing the PI3K/AKT pathway. J Cell Mol Med 2020;24: 11960–11971. doi: 10.1111/jcmm.15818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Weng Q, Han J, Chen J. Alantolactone suppresses human osteosarcoma through the PI3K/AKT signaling pathway. Mol Med Rep 2020;21: 675–684. doi: 10.3892/mmr.2019.10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao Z Han L Chen Y He F Sun B Kamar S, et al. . Hedgehog signalling in the tumourigenesis and metastasis of osteosarcoma, and its potential value in the clinical therapy of osteosarcoma. Cell Death Dis 2018;9: 701. doi: 10.1038/s41419-018-0647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doheny D, Manore SG, Wong GL, Lo HW. Hedgehog signaling and truncated GLI1 in cancer. Cells 2020;9: 2114. doi: 10.3390/cells9092114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang C Jing J Hu X Yu S Yao F Li Z, et al. . Gankyrin activates the hedgehog signalling to drive metastasis in osteosarcoma. J Cell Mol Med 2021;25: 6232–6241. doi: 10.1111/jcmm.16576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y, Yu P, Wang S, Jiang L, Chen F, Chen W. Crosstalk between Hh and Wnt signaling promotes osteosarcoma progression. Int J Clin Exp Pathol 2019;12: 768–773. [PMC free article] [PubMed] [Google Scholar]
- 40.Nagao-Kitamoto H Setoguchi T Kitamoto S Nakamura S Tsuru A Nagata M, et al. . Ribosomal protein S3 regulates GLI2-mediated osteosarcoma invasion. Cancer Lett 2015;356(2 Pt B): 855–861. doi: 10.1016/j.canlet.2014.10.042. [DOI] [PubMed] [Google Scholar]
- 41.Deng Q Li P Che M Liu J Biswas S Ma G, et al. . Activation of hedgehog signaling in mesenchymal stem cells induces cartilage and bone tumor formation via Wnt/β-catenin. Elife 2019;8: e50208. doi: 10.7554/eLife.50208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirotsu M Setoguchi T Sasaki H Matsunoshita Y Gao H Nagao H, et al. . Smoothened as a new therapeutic target for human osteosarcoma. Mol Cancer 2010;9: 5. doi: 10.1186/1476-4598-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao Z Jia Q Wu MS Xie X Wang Y Song G, et al. . Degalactotigonin, a natural compound from Solanum nigrum L. , inhibits growth and metastasis of osteosarcoma through GSK3β inactivation-mediated repression of the Hedgehog/Gli1 pathway. Clin Cancer Res 2018;24: 130–144. doi: 10.1158/1078-0432.CCR-17-0692. [DOI] [PubMed] [Google Scholar]
- 44.Tyagi A, Sharma AK, Damodaran C. A review on Notch signaling and colorectal cancer. Cells 2020;9: 1549. doi: 10.3390/cells9061549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li L Tang P Li S Qin X Yang H Wu C, et al. . Notch signaling pathway networks in cancer metastasis: A new target for cancer therapy. Med Oncol 2017;34: 180. doi: 10.1007/s12032-017-1039-6. [DOI] [PubMed] [Google Scholar]
- 46.Qin J Wang R Zhao C Wen J Dong H Wang S, et al. . Notch signaling regulates osteosarcoma proliferation and migration through Erk phosphorylation. Tissue Cell 2019;59: 51–61. doi: 10.1016/j.tice.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Zheng L, Conner SD. PI5P4Kγ functions in DTX1-mediated Notch signaling. Proc Natl Acad Sci U S A 2018;115: E1983–E1990. doi: 10.1073/pnas.1712142115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L, Wang J, Li JW, Zhao XW, Tian LF. LncRNA MEG3 inhibits proliferation and promotes apoptosis of osteosarcoma cells through regulating Notch signaling pathway. Eur Rev Med Pharmacol Sci 2020;24: 581–590. doi: 10.26355/eurrev_202001_20034. [DOI] [PubMed] [Google Scholar]
- 49.Jin H Luo S Wang Y Liu C Piao Z Xu M, et al. . miR-135b stimulates osteosarcoma recurrence and lung metastasis via Notch and Wnt/β-catenin signaling. Mol Ther Nucleic Acids 2017;8: 111–122. doi: 10.1016/j.omtn.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mödder UI, Oursler MJ, Khosla S, Monroe DG. Wnt10b activates the Wnt, Notch, and NFκB pathways in U2OS osteosarcoma cells. J Cell Biochem 2011;112: 1392–1402. doi: 10.1002/jcb.23048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moon H, Ro SW. MAPK/ERK signaling pathway in hepatocellular carcinoma. Cancers (Basel) 2021;13: 3026. doi: 10.3390/cancers13123026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med 2020; 19: 1997–2007. doi: 10.3892/etm.2020.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopes LJS, Tesser-Gamba F, Petrilli AS, de Seixas Alves MT, Garcia-Filho RJ, Toledo SRC. MAPK pathways regulation by DUSP1 in the development of osteosarcoma: Potential markers and therapeutic targets. Mol Carcinog 2017;56: 1630–1641. doi: 10.1002/mc.22619. [DOI] [PubMed] [Google Scholar]
- 54.Miao JH Wang SQ Zhang MH Yu FB Zhang L Yu ZX, et al. . Knockdown of galectin-1 suppresses the growth and invasion of osteosarcoma cells through inhibition of the MAPK/ERK pathway. Oncol Rep 2014;32: 1497–1504. doi: 10.3892/or.2014.3358. [DOI] [PubMed] [Google Scholar]
- 55.Burotto M, Chiou VL, Lee JM, Kohn EC. The MAPK pathway across different malignancies: A new perspective. Cancer 2014; 120: 3446–3456. doi: 10.1002/cncr.28864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lilienthal I, Herold N. Targeting molecular mechanisms underlying treatment efficacy and resistance in osteosarcoma: A review of current and future strategies. Int J Mol Sci 2020;21: 6885. doi: 10.3390/ijms21186885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Conforti F Pala L Bagnardi V De Pas T Martinetti M Viale G, et al. . Cancer immunotherapy efficacy and patients' sex: A systematic review and meta-analysis. Lancet Oncol 2018;19: 737–746. doi: 10.1016/S1470-2045(18)30261-4. [DOI] [PubMed] [Google Scholar]
- 58.Sun CY Zhang Z Tao L Xu FF Li HY Zhang HY, et al. . T cell exhaustion drives osteosarcoma pathogenesis. Ann Transl Med 2021;9: 1447. doi: 10.21037/atm-21-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Merchant MS Wright M Baird K Wexler LH Rodriguez-Galindo C Bernstein D, et al. . Phase I clinical trial of ipilimumab in pediatric patients with advanced solid tumors. Clin Cancer Res 2016;22: 1364–1370. doi: 10.1158/1078-0432.CCR-15-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paoluzzi L Cacavio A Ghesani M Karambelkar A Rapkiewicz A Weber J, et al. . Response to anti-PD1 therapy with nivolumab in metastatic sarcomas. Clin Sarcoma Res 2016;6: 24. doi: 10.1186/s13569-016-0064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Christie JD Appel N Canter H Achi JG Elliott NM de Matos AL, et al. . Systemic delivery of TNF-armed myxoma virus plus immune checkpoint inhibitor eliminates lung metastatic mouse osteosarcoma. Mol Ther Oncolytics 2021;22: 539–554. doi: 10.1016/j.omto.2021.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y Song Y Li P Li M Wang H Xu T, et al. . Downregulation of RIG-I mediated by ITGB3/c-SRC/STAT3 signaling confers resistance to interferon-α-induced apoptosis in tumor-repopulating cells of melanoma. J Immunother Cancer 2020;8: e000111. doi: 10.1136/jitc-2019-000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao J Wang M Li Z Chen J Yin Z Chang J, et al. . Interferon-α suppresses invasion and enhances cisplatin-mediated apoptosis and autophagy in human osteosarcoma cells. Oncol Lett 2014; 7: 827–833. doi: 10.3892/ol.2013.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whelan J Patterson D Perisoglou M Bielack S Marina N Smeland S, et al. . The role of interferons in the treatment of osteosarcoma. Pediatr Blood Cancer 2010;54: 350–354. doi: 10.1002/pbc.22136. [DOI] [PubMed] [Google Scholar]
- 65.Bielack SS Smeland S Whelan JS Marina N Jovic G Hook JM, et al. . Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: First results of the EURAMOS-1 good response randomized controlled trial. J Clin Oncol 2015;33: 2279–2287. doi: 10.1200/JCO.2014.60.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fu C, Zhou L, Mi QS, Jiang A. DC-based vaccines for cancer immunotherapy. Vaccines (Basel) 2020;8: 706. doi: 10.3390/vaccines8040706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He YT, Zhang QM, Kou QC, Tang B. In vitro generation of cytotoxic T lymphocyte response using dendritic cell immunotherapy in osteosarcoma. Oncol Lett 2016;12: 1101–1106. doi: 10.3892/ol.2016.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kawano M, Itonaga I, Iwasaki T, Tsumura H. Enhancement of antitumor immunity by combining anti-cytotoxic T lymphocyte antigen-4 antibodies and cryotreated tumor lysate-pulsed dendritic cells in murine osteosarcoma. Oncol Rep 2013;29: 1001–1006. doi: 10.3892/or.2013.2224. [DOI] [PubMed] [Google Scholar]
- 69.Kawano M Tanaka K Itonaga I Iwasaki T Miyazaki M Ikeda S, et al. . Dendritic cells combined with anti-GITR antibody produce antitumor effects in osteosarcoma. Oncol Rep 2015;34: 1995–2001. doi: 10.3892/or.2015.4161. [DOI] [PubMed] [Google Scholar]
- 70.Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: Treating cancer with specificity. Eur J Pharmacol 2018;834: 188–196. doi: 10.1016/j.ejphar.2018.07.034. [DOI] [PubMed] [Google Scholar]
- 71.Santos EDS Nogueira KAB Fernandes LCC Martins JRP Reis AVF Neto JBV, et al. . EGFR targeting for cancer therapy: Pharmacology and immunoconjugates with drugs and nanoparticles. Int J Pharm 2021;592: 120082. doi: 10.1016/j.ijpharm.2020.120082. [DOI] [PubMed] [Google Scholar]
- 72.Wang S Wei H Huang Z Wang X Shen R Wu Z, et al. . Epidermal growth factor receptor promotes tumor progression and contributes to gemcitabine resistance in osteosarcoma. Acta Biochim Biophys Sin (Shanghai) 2021;53: 317–324. doi: 10.1093/abbs/gmaa177. [DOI] [PubMed] [Google Scholar]
- 73.Frezzetti D Gallo M Maiello MR D'Alessio A Esposito C Chicchinelli N, et al. . VEGF as a potential target in lung cancer. Expert Opin Ther Targets 2017;21: 959–966. doi: 10.1080/14728222.2017.1371137. [DOI] [PubMed] [Google Scholar]
- 74.Xie L, Ji T, Guo W. Anti-angiogenesis target therapy for advanced osteosarcoma (Review). Oncol Rep 2017;38: 625–636. doi: 10.3892/or.2017.5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu Y Huang N Liao S Rothzerg E Yao F Li Y, et al. . Current research progress in targeted anti-angiogenesis therapy for osteosarcoma. Cell Prolif 2021;54: e13102. doi: 10.1111/cpr.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Assi T Watson S Samra B Rassy E Le Cesne A Italiano A, et al. . Targeting the VEGF pathway in osteosarcoma. Cells 2021; 10: 1240. doi: 10.3390/cells10051240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fioramonti M Fausti V Pantano F Iuliani M Ribelli G Lotti F, et al. . Cabozantinib affects osteosarcoma growth through a direct effect on tumor cells and modifications in bone microenvironment. Sci Rep 2018;8: 4177. doi: 10.1038/s41598-018-22469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang Q, Zhang S, Kang M, Dong R, Zhao J. [Retracted] Synergistic growth inhibition by sorafenib and cisplatin in human osteosarcoma cells. Oncol Rep 2021;46: 193. doi: 10.3892/or.2021.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park HJ Bae JS Kim KM Moon YJ Park SH Ha SH, et al. . The PARP inhibitor olaparib potentiates the effect of the DNA damaging agent doxorubicin in osteosarcoma. J Exp Clin Cancer Res 2018;37: 107. doi: 10.1186/s13046-018-0772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Franceschini N Gaeta R Krimpenfort P Briaire-de Bruijn I Kruisselbrink AB Szuhai K, et al. . A murine mesenchymal stem cell model for initiating events in osteosarcomagenesis points to CDK4/CDK6 inhibition as a therapeutic target. Lab Invest 2022;102: 391–400. doi: 10.1038/s41374-021-00709-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen C Guo Y Huang Q Wang B Wang W Niu J, et al. . PI3K inhibitor impairs tumor progression and enhances sensitivity to anlotinib in anlotinib-resistant osteosarcoma. Cancer Lett 2022; 536: 215660. doi: 10.1016/j.canlet.2022.215660. [DOI] [PubMed] [Google Scholar]
- 82.Yao P Lu Y Cai Z Yu T Kang Y Zhang Y, et al. . Research progress of exosome-loaded miRNA in osteosarcoma. Cancer Control 2022;29: 10732748221076683. doi: 10.1177/10732748221076683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu W Wang B Duan A Shen K Zhang Q Tang X, et al. . Exosomal transfer of miR-769-5p promotes osteosarcoma proliferation and metastasis by targeting DUSP16. Cancer Cell Int 2021;21: 541. doi: 10.1186/s12935-021-02257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao X, Wu Q, Gong X, Liu J, Ma Y. Osteosarcoma: A review of current and future therapeutic approaches. Biomed Eng Online 2021;20: 24. doi: 10.1186/s12938-021-00860-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ru JY, Cong Y, Kang WB, Yu L, Guo T, Zhao JN. Polymorphisms in TP53 are associated with risk and survival of osteosarcoma in a Chinese population. Int J Clin Exp Pathol 2015;8: 3198–3203. [PMC free article] [PubMed] [Google Scholar]
- 86.Wu J, Guo A, Li Q, Wang D. Meta-analysis of clinical significance of p53 protein expression in patients with osteosarcoma. Future Oncol 2017;13: 1883–1891. doi: 10.2217/fon-2017-0180. [DOI] [PubMed] [Google Scholar]