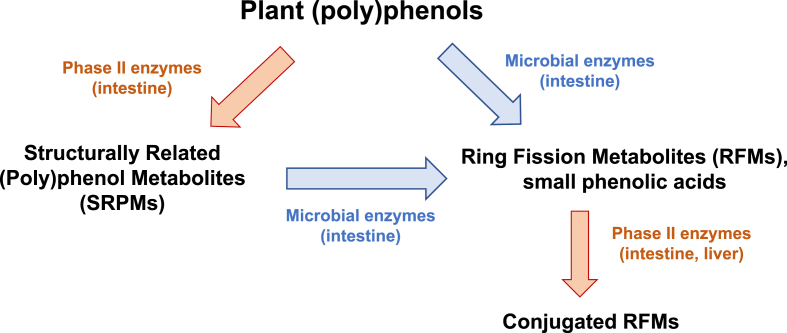
Fig. 1.
Metabolism of plant (poly)phenols ingested by humans and rodents. Depending on their chemical structures, (poly)phenols can be metabolized by: i) intestinal phase II enzymes to produce metabolites that maintain most of the original structure: Structurally Related (Poly)phenol metabolites (SRPMs), e.g. glucuronidated, sulfated, and methylated conjugates of the ingested (poly)phenols; ii) microbial enzymes that are able to breakdown the benzene rings to produce Ring Fission Metabolites (RFMs), e.g. small phenolic acids and other small molecules (γ-valerolactones, C6–C3 phenylpropanoic acids, C6–C2 phenylacetic acids, C6–C1 benzoic acids, hydroxyhippuric acid, etc.). These RFMs can be substrates for intestinal phase II enzymes producing their conjugated derivatives resulting in a diverse and complex mixture of compounds, including from larger (e.g. flavonoid conjugates) to smaller molecules (e.g. hydroxyhippuric acid).