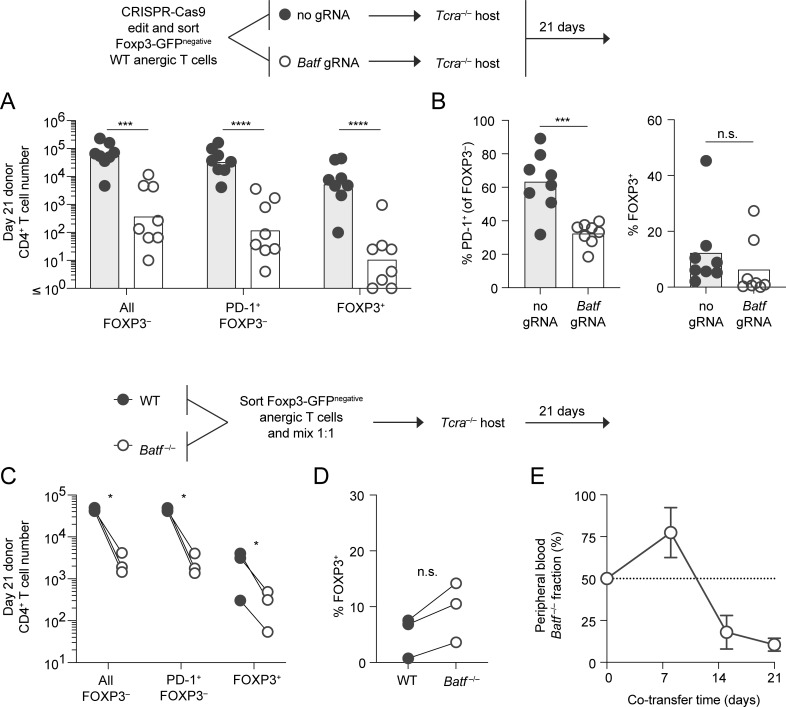
Figure 7.
Both conventional T cells and anergy-derived Treg cells rely on BATF for long-term survival following anergy reversal. (A) CD44high Foxp3(GFP)− CD73high FR4high (anergic) polyclonal CD4+ T cells were modified with CRISPR-Cas9 (with gRNA as indicated) and transferred into separate Tcra−/− recipient mice to facilitate anergy reversal. Recipient SLOs were then analyzed on day 21 after transfer for resulting conventional and Treg cell subpopulations as indicated (n = 8 mice, four independent experiments). Bars represent the geometric mean for the indicated cell phenotype (unpaired t test; ***P < 0.001, ****P < 0.0001). (B) Mean percentage of PD-1–expressing FOXP3− conventional (left) and FOXP3+ Treg (right) polyclonal donor T cells recovered from day 21 host mice following CRISPR-Cas9 treatment in the presence of Batf gRNA or no gRNA (sham control) as in A (unpaired t test; ***P < 0.001, n.s = not significant). n = 8 mice, in four independent experiments. (C–E) Anergic polyclonal CD4+ T cells were sorted from congenically marked WT and Batf−/− mice, mixed 1:1, and co-transferred into shared Tcra−/− recipients (n = 3 mice per group, one experiment). Donor-derived cell numbers based on phenotype (C) and (D) percentage of WT and Batf−/− donor-derived FOXP3+ Treg cells recovered from recipient SLOs on day 21 (paired t test; *P < 0.05, n.s. = not significant). (E) Fraction of donor CD4+ T cells in the peripheral blood that were Batf-deficient at the indicated time points (mean ± SD).