Abstract
Background
Angiotensin II (AII), has been suggested to promote muscle loss. Reducing AII synthesis, by inhibiting angiotensin converting enzyme (ACE) activity has been proposed as a method to inhibit muscle loss. The LACE clinical trial was designed to determine whether ACE inhibition would reduce further muscle loss in individuals with sarcopenia but suffered from low recruitment and returned a negative result. Polymorphic variation in the ACE promoter (I/D alleles) has been associated with differences in ACE activity and muscle physiology in a range of clinical conditions. This aim of this analysis was to determine whether I/D polymorphic variation is associated with muscle mass, strength, in sarcopenia or contributed to the lack of response to treatment in the LACE study.
Methods
Sarcopenic individuals were recruited into a 2x2 factorial multicentre double-blind study of the effects of perindopril and/or leucine versus placebo on physical performance and muscle mass. DNA extracted from blood samples (n = 130 72 women and 58 men) was genotyped by PCR for the ACE I/D polymorphism. Genotypes were then compared with body composition measured by DXA, hand grip and quadriceps strength before and after 12 months’ treatment with leucine and/or perindopril in a cross-sectional analysis of the influence of genotype on these variables.
Results
Allele frequencies for the normal UK population were extracted from 13 previous studies (I = 0.473, D = 0.527). In the LACE cohort the D allele was over-represented (I = 0.412, D = 0.588, p = 0.046). This over-representation was present in men (I = 0.353, D = 0.647, p = 0.010) but not women (I = 0.458, D = 0.532, p = 0.708). In men but not women, individuals with the I allele had greater leg strength (II/ID = 18.00 kg (14.50, 21.60) vs DD = 13.20 kg (10.50, 15.90), p = 0.028). Over the 12 months individuals with the DD genotype increased in quadriceps strength but those with the II or ID genotype did not. Perindopril did not increase muscle strength or mass in any polymorphism group relative to placebo.
Conclusion
Our results suggest that although ACE genotype was not associated with response to ACE inhibitor therapy in the LACE trial population, sarcopenic men with the ACE DD genotype may be weaker than those with the ACE I/D or II genotype.
Introduction
The normal human growth cycle includes periods of muscle growth, muscle maintenance and muscle loss [1]. As people live to a greater age through improved environmental conditions and medical therapies, the period of loss is prolonged. Loss of muscle mass and strength in older age is termed sarcopenia [2], which is associated with major adverse consequences including falls, dependency and the need for care, hospital admission, prolonged hospital stay and earlier death [3]. As muscle mass and strength are governed by environmental, genetic and epigenetic factors, the combination of these will determine whether an individual becomes sarcopenic, and how rapidly the condition will deteriorate. The genetic factors contributing to sarcopenia are likely to include those that contribute to muscle mass and strength in adulthood and identifying such factors and pathways will aid the identification of therapeutic approaches to prevent, mitigate or help reverse sarcopenia.
The renin-angiotensin system has gained significant attention as a potential target pathway to treat sarcopenia [4–8]. Angiotensin II (AII) promotes the skeletal muscle catabolic pathway (e.g., MuRF1) in vitro [9] and inhibits IGF-1 production [10]. Infusion of angiotensin into mice promotes skeletal atrophy [11]. In one small before and after clinical study of patients with heart failure, 6 months treatment with ACEi or AII antagonists increased exercise performance [12]. In cross sectional studies of individuals on antihypertensive therapies, ACEi usage associated with preservation of muscle mass and strength [13, 14]. Other studies have combined ACEi with exercise in both individuals with COPD and women with sarcopenia to attempt to identify combinatorial effects but the results of these trials do not provide a consistent picture of increased effectiveness of exercise interventions in those receiving ACEi [15–17].
These data suggest that reducing ACE activity and thereby AII could preserve muscle mass and strength in older individuals and one interventional study has supported this suggestion [18]. However, other trials in patients with COPD [4] and in patients with hypertension [19] have not shown a beneficial effect of ACEi. We recently conducted and published a multicentre 2 x 2 factorial RCT of leucine and ACE inhibitors in older people with sarcopenia (the LACE trial) which also showed no overall benefit from perindopril (an ACEi) therapy [20]. Whilst recruitment to this trial was low (as discussed in [21]) resulting in early termination by the funder, a meta-analysis of similar trials also showed a lack of effect, supporting the findings [20].
Studies of genetic variation show that the relationship between ACE activity and strength is not as simple as lower ACE activity associating with increased strength. One well-studied ACE promoter polymorphism, the insertion (I)/deletion (D) polymorphism, affects ACE synthesis with the D allele associated with greater ACE synthesis and higher circulating ACE activity [22]. However, contrary to the effects of AII on muscle mass and strength, several studies associate the ACE D allele with greater muscle mass and strength. For example, the D allele has been associated with increased performance in power sports [23], with increased strength following exercise training [24], and with increased strength in COPD patients [25]. Conversely, the I allele is associated with endurance [26, 27]. However, not all studies show the same enrichment of the D allele with strength in elite sports [28]. The effects of this polymorphism on muscle performance in the older population is unclear. In a study of 65-year-old Koreans the II genotype was associated with increased fatigue resistance [29]. In an Indonesian population of over 60 years the DD genotype was associated with reduced muscle mass [30] and in a Spanish cohort, individuals carrying the I allele had greater hand-grip strength than those with the DD genotype [31]. Conversely, in a large study of older Americans, (70–79 years) who continued to exercise, individuals with the II phenotype were more likely to show a loss of functional capacity [32]. One recent study of older individuals failed to identify any differences in lower limb strength normalised for body weight [33] and one further study failed to see any effect of the ACE I/D polymorphism on the exercise response in women over 60 [34].
These effects of the I/D polymorphism could be explained by variations in muscle fibre proportions. Skeletal muscle can be broadly categorised into two major fibre types (oxidative type I and more glycolytic type II). Type I fibres are used in endurance activities, whereas type II fibres are associated with power activities. In untrained volunteers, the D allele is associated with a greater proportion of type II fibres, with the I allele associated with a greater proportion of type I fibres [35], and treatment of patients with ACEi for 6 months increases MHC I (Myh7) expression [12]. Thus, over extended periods of time, higher ACE activity within the normal range, appears to promote a higher type II fibre proportion and so higher strength. However, more recent data have suggested that in Japanese individuals the opposite association between ACE genotype and muscle fibre type exists [36].
Type II fibres are more sensitive to both anabolic and catabolic stimuli than type I fibres [37, 38]. This higher sensitivity may contribute to the general loss of type II fibres that occurs in older individuals. However, this shift towards type I fibres is complicated by a shift in the opposite direction (increased proportion of type II fibres) that accompanies reduced physical activity and is seen in many patients with chronic disease [39].
It is therefore difficult to predict the effect of ACE genotype on the likelihood of sarcopenia as DD individuals may have started with a larger muscle mass and greater strength with a higher type II fibre composition that is more susceptible to loss. The effects of these polymorphisms may have contributed to the findings of the LACE study by affecting the muscle mass of individuals before the study or by modifying the response to therapy. In this study therefore, we analysed the genotypes of individuals with sarcopenia, enrolled into the LACE study to determine any association of ACE genotype with strength or muscle mass. We also determined the effect of genotype on the change in muscle mass, strength and physical performance 1 year later in those who completed the trial.
Materials and methods
Participants and physiological analysis
Individuals over the age of 70 with sarcopenia according to the EWGSOP definition (2010) [40] were recruited at 14 UK centres between April 2016 and December 2019 to a 2x2 factorial trial of leucine and/or ACE inhibition (trial registration ISRCTN90094835). Full recruitment criteria and methodology are described in [20]. The trial was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments and approved by the East of Scotland NHS research ethics committee (approval 14/ES/1099) and the UK Medicines and Healthcare Regulatory Authority (EudraCT number 2014-003455-61; Clinical Trial Authorisation number 36888/0001/001-0001). The study was a double-blind randomised controlled trial and sample preparation and analysis was carried out without any access to information that could identify the individuals involved. The trial protocol and primary outcomes have been published [20, 41]. The CONSORT diagram for the study with details of recruitment and dropout from the trial is published [20, 41]. Participants invited to attend for a screening visit had their age, weight and height measured and bioimpedance was used to measure overall muscle mass as part of the recruitment process. Bioimpedance was used at this stage to reduce the total number of DXA scans required. Individuals conforming to the EWGSOP guidelines as sarcopenic were recruited and attended a baseline visit. At this visit, their appendicular body composition (muscle mass, fat mass and bone mass) was determined by DXA and their physical performance measured. Physical performance measures were 6-minute walk distance (6MW) and short physical performance battery (SPPB), grip strength (measured using a hand-held Jamar dynamometer) and leg strength (Quadriceps Maximum Voluntary Contraction QMVC, measured as isometric voluntary knee extension with a Lafayette 01165 dynamometer Lafayette Instrument, Lafayette, IN, USA). Measurements were taken with the participant seated, the knee joint at 90 degrees, and with a non-elastic strap running between the chair and the ankle to restrain the dynamometer. The patients were randomised to placebo, leucine, perindopril or both leucine and perindopril at this baseline visit. Appendicular body composition and all physical performance measures were repeated 12 months later for those completing the trial. The LACE trial did not attempt to calibrate DXA readings between different sites or machines and this may have increased the noise inherent in the DXA results. However, each participant had their baseline and 12-month DXA scans performed on the same machine, removing this as a source of error from the longitudinal data. QMVC was divided by leg muscle mass to give a normalised quadriceps strength (kg/kg). Proportionate change was calculated as 12-month value/baseline value for each variable with longitudinal data.
The original study was powered to show difference in SPPB, but the study was terminated early due to poor recruitment. Of the 145 individuals recruited to the study 15 dropped out prior to a 3 month visit and their baseline samples were not retained for this study. All available samples were included in this analysis.
DNA analysis
Whole blood samples were taken at the first study visit and frozen. DNA was extracted using the QIAamp DNA blood mini kit according to the manufacturer’s instructions. DNA genotyping was performed by PCR using the primers ACE I/D-F CTGGAGACCACTCCCATCCTTTCT and ACE I/D-R GATGTGGCCATCACATTCGTCAGAT as described [42]. The reaction mix was made using HOTstarTaq DNA polymerase (Qiagen) included 2pmol of each primer. The samples were amplified for 40 cycles using a cycle of 95C for 1 min 58 C for 1 min and 72 C for 1 min in a VertiPro 96 well thermocycler. The PCR products were separated by agarose gel electrophoresis through a 1.5% agarose gel, stained with SYBRsafe and visualised on a GelDoc imager (Biorad). All available samples from the study were included.
Statistical analysis
The original study was a double-blind randomised controlled trial and sample preparation and analysis for this work was carried out without any access to information that could identify the individuals involved. Statistical analysis was performed in Aabel 3.0 and SPSS with the exception of the Chi squared test. Normality was determined by Shapiro-Wilk test, with differences between groups determined by Mann-Whitney U test for data that was not normal distributed and Student’s T-test for normally distributed data. Changes in strength at baseline and follow-up were determined by Wilcoxon matched-pairs signed rank test in Aabel 3.0. Values are given as median (interquartile range) for non-parametric data or as mean ± SD for normally distributed data. The threshold for statistical significance (alpha) was taken as 0.05. All available data was used for each comparison and no manipulations were performed to include missing values for individual tests. As the analysis here is exploratory, no corrections have been made for multiple testing. Reasons for loss to follow-up is detailed in [20, 41].
Results
All individuals enrolled into the LACE study were sarcopenic as defined by EWGSOP and the demographics of the cohort are given in Table 1. Therefore, to establish an allele frequency for the ACE genotype in the UK population, the genotypes of controls from 13 published studies with a UK only population were averaged [43–55]. The ages of individuals and the study from which they are taken, and the details of the cohort used are given in S1 Table. This identified a total of 9909 genotypes (Table 2) and showed that the relative frequency of the alleles was I = 0.473 and D = 0.527. In the entire LACE cohort there was a significant over representation of the D allele (LACE population I = 0.411, D = 0.588, Chi2 = 3.97, p = 0.046). This difference was due to an excess of the D allele in the men but not the women (LACE men; I = 0.353, D = 0.647, Chi2 = 6.70, p = 0.010, LACE women; I = 0.458, D = 0.542, Chi2 = 0.14, p = 0.708, Table 2). Restricting the UK population to just men using the studies where sex and genotype were identifiable, gave a genotype frequency of I = 0.477, D = 0.523. However, this was from a smaller number of individuals (n = 710, II = 157, ID = 364, DD = 189) and the LACE male distribution was even further from the allele frequencies in this group than from the whole UK cohort (Chi2 = 7.10, p = 0.008). Restricting the UK cohort to those studies of individuals with an average age over 75 (II = 144, ID = 308, DD = 204) gave allele frequencies of I = 0.454, D = 0546. The men in the LACE cohort were still significantly different to this cohort (Chi2 = 4.85, p = 0.028).
Table 1. Demographics of the cohort.
| Women | Men | |
|---|---|---|
| number | 72 [52] | 58 [47] |
| Age (years) | 78 (75, 82) | 77 (74, 84) |
| weight (kg) | 63.0 (56.9, 71.0) | 80.2 (73.5, 90.1) |
| height (cm) | 157 ± 6 | 171 ± 7 |
| BMI (kg/m2) | 26.1 (23.2, 28.2) | 27.2 (25.4, 30.5) |
| SARC-F | 3 (4, 5) | 4 (3, 4) |
| muscle mass (kg/m2) | 5.74 (5.44, 5.99) | 7.23 ± 0.64 |
| upper limb fat mass (kg) | 3.14 (2.45, 3.78) | 2.64 (2.23, 3.17) |
| upper limb muscle mass (kg) | 3.38 ± 0.56 | 5.92 ± 0.96 |
| lower limb fat mass (kg) | 9.24 (7.52, 11.88) | 6.48 (5.41, 8.72) |
| lower limb muscle mass (kg) | 10.66 ± 1.63 | 15.87 ± 2.46 |
| SPPB baseline | 7.0 (5.8, 9.0) | 7.5 (5.0, 9.0) |
| SPPB 12 months | 8.0 (5.0, 9.3) | 8.0 (6.0, 9.0) |
| Grip strength baseline (kg) | 13.6± 3.6 | 22.4 ± 5.4 |
| Grip strength 12 months (kg) | 15.0 ± 3.3 | 23.4 ± 5.9 |
| QMVC baseline (kg) | 9.9 (6.7, 13.0) | 15.7 (11.9, 21.1) |
| QMVC 12 months (kg) | 10.4 (7.6, 13.4) | 15.0 (12.0, 18.5) |
Numbers given in square brackets are the number of individuals completing the trial. BMI: body mass index, SARC-F: Strength, assistance with walking, rising from a chair, climbing stairs, and falls questionnaire score, SPPB: Short Physical Performance Battery score: QMVC: Quadriceps Maximal Voluntary contraction.
Table 2. Genotypes of the cohort.
| Genotype | |||||||
|---|---|---|---|---|---|---|---|
| Group | II | ID | DD | I | D | Chi2 | P Value |
| Controls | 2200 | 4967 | 2742 | 0.473 | 0.527 | ||
| LACE | 21 (29) | 65 (65) | 44 (36) | 0.411 | 0.588 | 3.97 | 0.046 |
| Women | 15 (16) | 36 (36) | 21 (20) | 0.458 | 0.542 | 0.14 | 0.708 |
| Men | 6 (13) | 29 (29) | 23 (16) | 0.353 | 0.647 | 6.70 | 0.010 |
Predicted numbers using the control allele frequencies are given in brackets
Chi2 was calculated on allele number (degrees of freedom = 1)
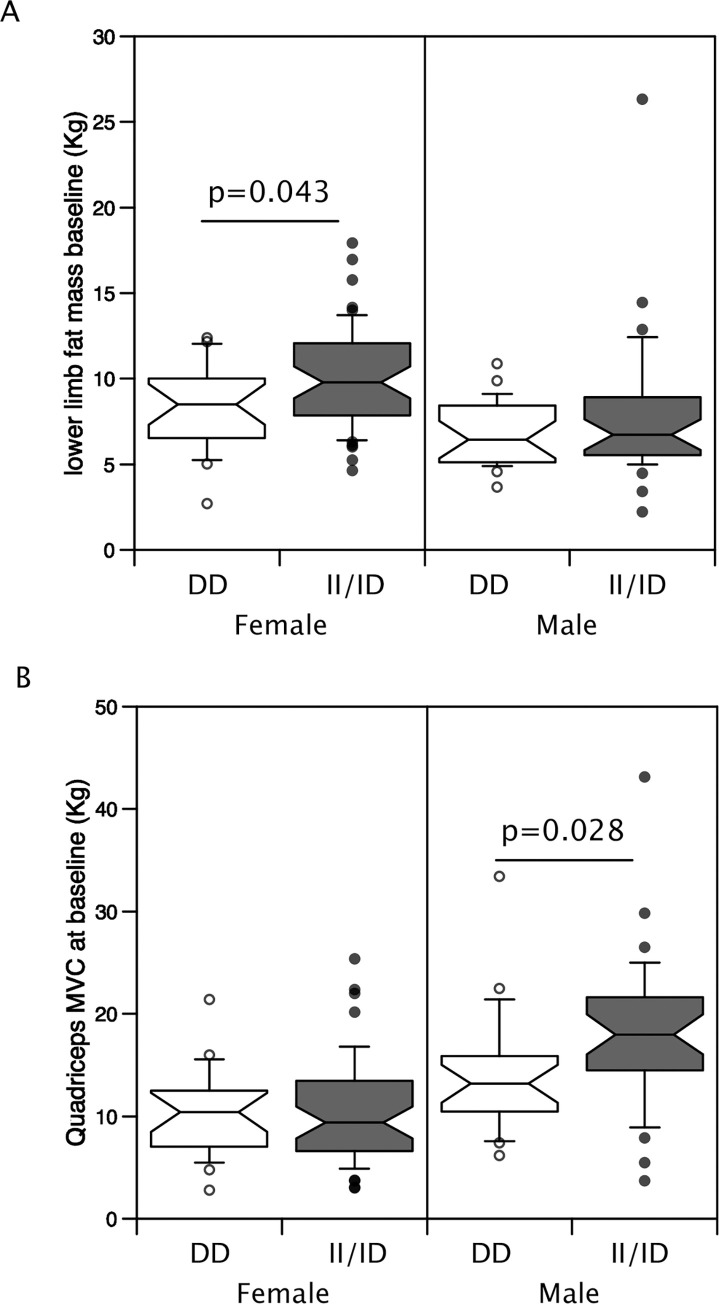
Given the difference in body composition and strength between men and women, the sexes were analysed separately. Furthermore, due to the small number of II homozygous individuals, the cohort was analysed as those possessing the minor (I) allele and DD individuals. In women only, the I allele was associated with higher fat mass in the lower limbs but not with upper or lower limb muscle mass or strength (Table 3, Fig 1). This difference in fat mass was not seen at 12 months in the individuals who completed the trial.
Table 3. Baseline body mass composition and muscle physiology in the different genotypes split by sex.
| Women | Men | |||||
|---|---|---|---|---|---|---|
| DD | ID/II | P value | DD | ID/II | P value | |
| n | 21 | 51 | 23 | 35 | ||
| Weight (kg) | 62.87 ± 12.51 | 64.45 ± 10.56 | 0.780 | 82.00 (73.70, 90.05) | 80.20 (73.55, 88.50) | 0.937 |
| BMI (kg/m2) | 25.06 (21.87, 27.04) | 26.16 (24.09, 28.60) | 0.232 | 28.41 (26.09, 30.11) | 27.11 (25.27, 30.40) | 0.567 |
| Height (m) | 157 (154, 163) | 157 (152, 161) | 0.289 | 169 (167, 174) | 173 (167, 177) | 0.373 |
| muscle mass (kg/m2) | 5.59 (5.29, 6.00) | 5.79 (5.53, 5.98) | 0.295 | 7.43 (6.79, 7.62) | 7.17 (6.82, 7.79) | 0.855 |
| upper limb fat mass (kg) | 2.73 (2.23, 3.51) | 3.21 (2.26, 3.83) | 0.511 | 2.52 (2.03, 3.02) | 2.78 (2.28, 3.25) | 0.131 |
| lower limb fat mass (kg) | 8.51 (6.54, 10.02) | 9.79 (7.85, 12.07) | 0.043 | 6.45 (5.20, 8.11) | 6.74 (5.54, 8.89) | 0.666 |
| upper limb muscle mass (kg) | 3.41 (3.09, 3.79) | 3.35 (2.92, 3.80) | 0.511 | 5.66 (5.09, 6.49) | 5.96 (5.15, 6.53) | 0.435 |
| lower limb muscle mass (kg) | 10.76 ± 1.22 | 10.62 ± 1.78 | 0.712 | 16.00 ± 1.63 | 15.79 ± 2.91 | 0.749 |
| SPPB baseline | 7.0 (6.0, 9.0) | 7.0 (5.0, 9.0) | 0.975 | 8.0 (6.0, 9.0) | 7.0 (5.0, 9.0) | 0.315 |
| Grip strength baseline (kg) | 13.9 ± 3.6 | 13.5 ± 3.6 | 0.593 | 22.8 ± 4.1 | 22.1 ± 6.1 | 0.595 |
| QMVC baseline (kg) | 10.4 (7.1, 12.4) | 9.4 (6.6, 13.5) | 0.796 | 13.2 (10.5, 15.9) | 18.0 (14.5, 21.6) | 0.028 |
| QMVC/leg muscle mass (kg/kg) | 0.93 (0.67, 1.19 | 0.93 (0.65, 1.19) | 1.000 | 0.78 (0.63, 1.15) | 1.18 (0.86,1.33) | 0.035 |
BMI: body mass index, SPPB: Short Physical Performance Battery score: QMVC: Quadriceps Maximal Voluntary contraction. Normally distributed data are presented as mean ± SD with significance of differences determined by t-test. Non-parametric data are presented as median (inter quartile range) and the significance of differences was determined by Mann Whitney U test
Fig 1. Effect of ACE genotype on lower limb fat and muscle strength.
Limb composition was determined by DXA in 72 women and 58 men. The individuals were genotyped for the ACE I/D polymorphism and the effect of polymorphic variation on body composition and strength was determined. The II/ID genotypes were associated with a higher lower limb fat mass than the DD genotype in women but not in men (A). In men but not women, the II/ID genotypes were associated with greater quadriceps muscle strength than the DD genotype. (p Values calculated by Mann-Whitney U test).
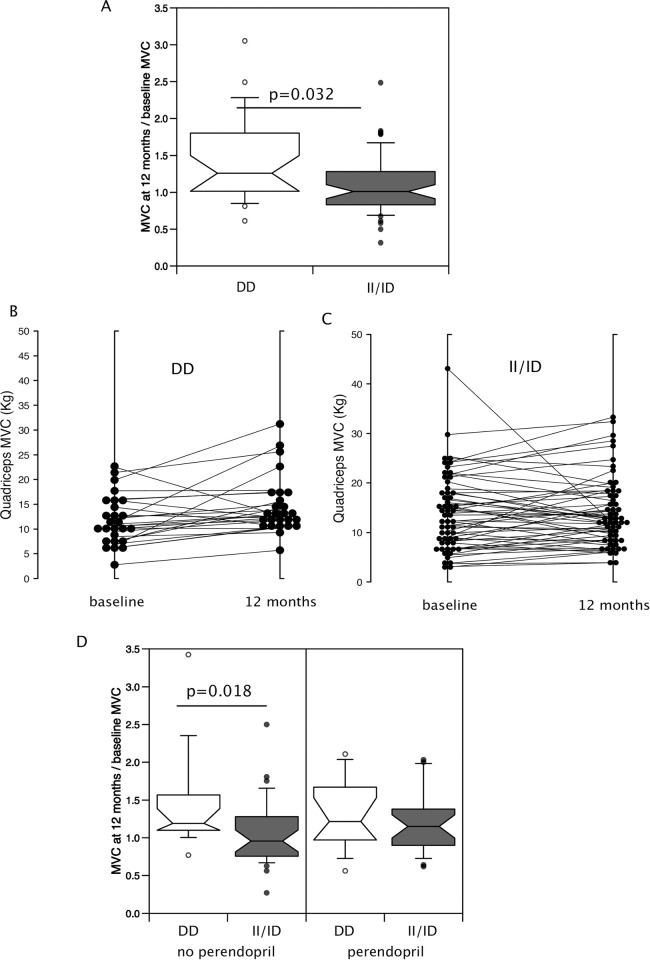
In men, presence of the minor allele was associated with higher QMVC both as a raw value (II/ID median 18.0 kg IQR (14.5, 21.6) vs DD 13.2 kg (10.5, 15.9) p = 0.028 Fig 1, Table 3) and when normalised to leg muscle mass determined by DXA (II/ID 1.18 kg/kg (0.86,1.33) vs DD 0.78 kg/kg (0.63, 1.15) Table 3, p = 0.035). However, there was no difference in lower limb muscle mass between the genotypes. Using proportionate change in muscle strength over 12 months to allow the combination of both men and women showed that the DD genotype was associated with a greater average increase in muscle strength over the 12-month period (% change in QMVC: II/ID median = 3.8% (-21.1%- 30.6%), DD median = 20.8% (8.8%-66%), p = 0.032, Fig 2A). To confirm this analysis comparing QMVC at baseline and follow-up using a Wilcoxon matched-pairs signed rank test within the genotypes showed that the DD individuals increased in strength (baseline 10.8 kg (7.6, 15.8), vs 12 months 12.8 kg (10.5, 17.4), p = 0.003) over the period of the trial whereas the II/ID individuals did not (baseline 13.0 kg (8.1, 18.1), vs 12 months 12.2 kg (8.6, 17.8), p = 0.504, Fig 2B and 2C). However, there was no accompanying change in leg muscle mass (DD individuals; baseline 14.6 kg (11.5, 15.9) vs 12 months 14.3 kg (11.3, 15.4) p = 0.322, II/ID individuals; baseline 12.1 kg (10.4, 15.5) vs 12 months 12.5 kg (10.5, 15.9) p = 0.808).
Fig 2. Effect of genotype on change in strength over 12 months of the LACE trial.
Proportionate strength was determined by dividing MVC at month 12 by MVC at baseline allowing us to combine data from men and women in the study. (A) Individuals with the DD genotype had a higher proportionate strength at month 12 than individuals with the II/ID genotype (Mann-Whitney U test). (B and C) Comparison of the baseline and 12-month MVC showed that the DD individuals (B) increased in strength over the course of the trial (p = 0.003 Wilcoxon-signed rank test) whereas there was no change in strength in the II/ID individuals (C). There was no effect of perindopril on proportionate strength in either the DD or II/ID genotypes (D).
To determine if the increase in muscle strength was a consequence of the treatment with perindopril, we compared QMVC at 0 and 12 months in II/ID and DD individuals who completed the study. This analysis showed that, consistent with the overall trial data, perindopril did not cause the increase in strength in the DD genotype individuals or the II/ID individuals as specified groups. Wilcoxon matched-pairs analysis showed that the DD individuals on perindopril did not increase their strength over 12 months (change in QMVC = 2.55 kg (-0.35, 4.15) p = 0.196) but those not on perindopril did (change in QMVC = 1.8 kg (1.2, 6.6), p = 0.008). However, strength did not increase in II/ID individuals whether on perindopril (change in QMVC = 1.45 kg (-2.3, 3.9), p = 0.247), or not (change in QMVC = -0.4 kg (-2.6, 3.3), p = 0.860). In the absence of perindopril, the increase in proportionate strength was larger in individuals with the DD than the II/ID genotype but there was no difference in the presence of perindopril (Fig 2, Table 4). No differences were seen in the change in QMVC between baseline and follow-up in either DD or II/ID genotypes in response to leucine.
Table 4. Effect of perindopril on muscle performance based on genotype.
| DD | ID/II | |||||
|---|---|---|---|---|---|---|
| perindopril | No perindopril | P value | perindopril | No perindopril | P value | |
| n | 15 | 17 | 30 | 37 | ||
| f/m | 5/10 | 8/9 | 17/13 | 22/15 | ||
| SPPB baseline | 8 (6, 9) | 7 (6, 8) | 0.635 | 8 (5, 9) | 7 (5, 9) | 0.896 |
| SPPB 12 months | 9 (5, 10) | 9 (6, 9) | 0.710 | 8 (4, 9) | 8 (6, 9) | 0.246 |
| Change in SPPB | 0 (-1, 2) | 1 (-1, 2) | 0.602 | 0 (-2, 1) | 1 (0, 2) | 0.017 |
| Proportionate upper limb muscle mass at 12 months | 1.01 (0.94, 1.05) | 1.01 (0.97, 1.05) | 0.821 | 1.03 (0.98, 1.07) | 1.00 (0.96, 1.04) | 0.303 |
| Proportionate lower limb muscle mass at 12 months | 1.00 (0.99, 1.03) | 1.01 (0.99, 1.05) | 0.545 | 1.01 (0.95, 1.05) | 0.99 (0.96, 1.02) | 0.692 |
| Proportionate grip strength at 12 months | 0.97 (0.90, 1.19) | 1.04 (0.92, 1.32) | 0.423 | 1.07 ± 0.33 | 1.01 (0.93, 1.11) | 0.898 |
| Proportionate QMVC at 12 months | 1.21 (0.97, 1.67) | 1.19 (1.10,1.57) | 0.667 | 1.15 (0.90, 1.38) | 0.96 (0.76, 1.27) | 0.217 |
SPPB: Short Physical Performance Battery score: QMVC: Quadriceps Maximal Voluntary Contraction. Data are presented as median (inter quartile range) and the significance of differences was determined by Mann Whitney U test
Finally, we compared the effect of perindopril on physical performance measured by the short physical performance battery (SPPB). In those with the II/ID genotype there was a median increase in SPPB of 1 point over the 12 months in the absence of perindopril but in the presence of perindopril median change in SPPB was 0 points (Table 4). In the DD genotype group, there was no change in SPPB at 12 months. Leucine had no effect on SPPB in either DD or II/ID genotypes.
Discussion
Our data show that, in the LACE cohort of sarcopenic individuals, the number of men carrying the I allele (II and ID combined) was lower than predicted from the general UK population. The I allele frequency in men in our study is 0.353 compared to the expected 0.474. Furthermore, we found that II/ID men had greater muscle strength than those with the DD genotype. This observation is opposite to the effects of the I/D polymorphism seen in the non-sarcopenic population where the D allele is found enriched in power athletes and is associated with greater muscle mass and strength in a number of diverse cohorts including those representing elite athletes, normal healthy individuals and patients with chronic obstructive pulmonary disease [23–25]. The reduction in the number of men carrying the I allele could be explained by one of three main mechanisms, survivor bias, recruitment bias or a resistance to sarcopenia for individuals with at least one I allele. A survivor or recruitment bias due to II males having died or been too frail to participate is unlikely given the body of literature indicating the association of the D allele with both morbidity and mortality [56–62]. However, although comparison with published UK studies where the individuals had average age over 75 did not affect our results, a recent meta-analysis of studies including centenarians, and older individuals as well as younger controls suggests a survivor advantage for DD individuals but not for ID individuals [63]. For this bias to account for our findings on the disproportionate number of DD men, the survivor effect would need to be greater and/or observed earlier in men as our observation of increased DD individuals was restricted to men. Given the size of our study this finding needs to be replicated in a larger study. It is also possible that recruitment bias occurred as a consequence of differences in the effects of EWGSOP algorithm in men and women. Rather, our finding would be consistent with II males being relatively resistant to sarcopenia, and therefore under-recruited. This possibility is consistent with the original hypothesis that lowering ACE activity would associate with increased muscle strength but if this was the major mechanism, it might be expected that further lowering ACE activity would have increased muscle strength and this did not happen. An alternative mechanism that could contribute to this resistance would be the higher type I fibre proportion associated with the I allele compared to D allele and the relative insensitivity of type I fibres to atrophy compared to type II fibres [35]. Therefore, individuals with the II genotype could start with a lower muscle mass and strength than their DD counterparts but be more likely to maintain muscle mass and strength and so less likely to become sarcopenic. The ability to maintain physical performance/endurance because of the greater proportion of type I fibres may also contribute to greater relative physical activity over time helping to maintain muscle mass. A reduced rate of muscle loss would also account for the reduced strength seen in the sarcopenic DD men compared to those carrying the I allele. Again, this reduced strength in DD men is counter to the situation in non-sarcopenic populations that have been studied which show higher strength in those with the DD genotype.
We did not observe either of these effects in women. Previous studies have shown that women have a higher proportion of type I fibres in the quadriceps compared to men [64, 65] suggesting that the effects of the II genotype may make a smaller difference to the overall proportion of fibres in women than in men. It is also possible that the effects of AII are enhanced by testosterone. For example, testosterone has been shown to contribute to the AII dependent increase in blood pressure in male rats [66], but any such cross talk in skeletal muscle remains to be established.
The LACE trial, from which these samples were taken, did not report any effect of perindopril on muscle mass or strength and a meta-analysis performed following the trial also concluded that ACE inhibition did not increase muscle mass or strength. In this analysis we show that there is no positive effect of ACE inhibition either in individuals with the II/ID genotypes or the DD genotypes although we did see an increase in muscle strength in DD individuals enrolled in the trial, whether or not they were on perindopril or leucine. The presence of this increase in strength may have been a consequence of a combination of the two agents that would have been identifiable had the trial been allowed to recruit its full cohort, a consequence of altered activity as a response to being recruited to a trial or potentially a type II error given the size of the trial. The reason that it is only observed in the DD group could suggest either that this group is most likely to respond to both activity and potentially to drug therapies due to a higher proportion of type II fibres or that as these individuals started with a lower QMVC there was a greater response to any increase in activity. Physical activity was not measured in this study. Whether trial recruitment acted as a trigger to increase activity would not have been detectable as it would not be possible to quantify pre-recruitment activity.
Consistent with a change in behaviour, individuals carrying the I allele showed an increase in SPPB by 1 point over the 12 months. Both changes (increased strength in DD individuals and increased SPPB in II/ID individuals) only occurred in the absence of perindopril. It is not clear why this change was restricted to those not on perindopril but given the drop-out rate was higher for those on perindopril than those not taking perindopril, it is possible that adverse responses to perindopril treatment reduced the effect of being recruited to a trial.
The LACE trial and a meta-analysis of available randomised controlled trials did not identify any effect of ACEi on muscle mass or strength (33), one possible reason for this is that the trial did not combine pharmacological treatment with exercise. In support of this possibility some studies have suggested that exercise responses are larger in patients taking ACEi than in those not taking ACEi [67] and some but not all animal studies have shown a that ACEi increases the gains from exercise [68, 69]. However, several randomised control trials of exercise have not found support for this proposal. Indeed, in patients with COPD combining ACEi with exercise reduced the efficacy of the exercise program (measured as peak work rate [16]) and in healthy volunteers, ACEi impaired lean muscle mass gain, reduced left atrial volume and reduced haemoglobin content [70]. Furthermore, in older individuals with functional impairment there was also no increased positive effect of ACEi with exercise [5]. The effects of genotype were not addressed in these studies, but the findings from our study would be most consistent with ACEi reducing any effect as the only improvements we saw (increase in SPPB in II/ID individuals and increase in strength in DD individuals) were in the group not taking perindopril.
Limitations of this study
This study is limited due to its very small sample size and therefore requires repeating in a larger study. The trial also only recruited individuals with sarcopenia making us reliant on allele frequencies from previous studies rather than a control cohort recruited at the same time and from the same communities. To overcome this latter problem, we identified studies with control cohorts of UK individuals to calculate an allele frequency that was representative of the general UK population. Furthermore, as the group sizes are small and the study was a 2x2 factorial trial it is possible that there was an effect of perindopril on strength in the II/ID group, but this is too small to detect in this study and is complicated either by the treatment with leucine or that inclusion in the trial leads to altered physical activity.
In conclusion our analysis suggests that sarcopenic men with the DD ACE genotype may be weaker than men with at least one I allele. We speculate that this is due to differences in the fibre types that have been shown to occur with the differences in genotype and the higher sensitivity of type II fibres to atrophy than type I fibres. The data imply that genotype does not account for the lack of effect of perindopril that we observed in the LACE trial. However, if the basis of the effects of ACE genotype is due to long-term effects of lower ACE levels on fibre type, it is possible that one year of treatment is not sufficient to alter the fibre proportions sufficiently to reduce the loss of strength. It is also possible that the number of type II fibres has been reduced to such an extent that fibre shift is no longer a viable route to increase muscle function.
Supporting information
(DOCX)
(XLSX)
Acknowledgments
The authors would also thank the efforts of all the research nurses and other site research staff who recruited participants to the trial, all the participants, and all the staff of the Tayside Clinical Trials Unit for their support of the trial.
Data Availability
The data are included in an excel sheet as supplementary material.
Funding Statement
The LACE trial (project reference 13/53/03) is funded by the Efficacy and Mechanism Evaluation (EME) Programme, an MRC and NIHR partnership. The Principal Investigator of the award was MW with PK, AS, AA, PD as co applicants. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The views expressed in this publication are those of the authors and not necessarily those of the MRC, NIHR or the Department of Health and Social Care. AAS, TA, and MDW acknowledge support from the NIHR Newcastle Biomedical Research Centre. AA acknowledges support from the Health Services Research Unit, which is core funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorate. The authors acknowledge support from the NIHR Ageing Clinical Research Network and the NHS Scotland Support for Science programme. These organisations had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Melton LJ 3rd, Khosla, Crowson CS, O’Connor MK, O’Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48(6):625–30. [PubMed] [Google Scholar]
- 2.Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393(10191):2636–46. doi: 10.1016/S0140-6736(19)31138-9 [DOI] [PubMed] [Google Scholar]
- 3.Han A, Bokshan SL, Marcaccio SE, DePasse JM, Daniels AH. Diagnostic Criteria and Clinical Outcomes in Sarcopenia Research: A Literature Review. J Clin Med. 2018;7(4):70. doi: 10.3390/jcm7040070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shrikrishna D, Tanner RJ, Lee JY, Natanek A, Lewis A, Murphy PB, et al. A randomized controlled trial of angiotensin-converting enzyme inhibition for skeletal muscle dysfunction in COPD. Chest. 2014;146(4):932–40. doi: 10.1378/chest.13-2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sumukadas D, Band M, Miller S, Cvoro V, Witham M, Struthers A, et al. Do ACE inhibitors improve the response to exercise training in functionally impaired older adults? A randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2014;69(6):736–43. doi: 10.1093/gerona/glt142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumukadas D, Witham MD, Struthers AD, McMurdo ME. Ace inhibitors as a therapy for sarcopenia—evidence and possible mechanisms. J Nutr Health Aging. 2008;12(7):480–5. doi: 10.1007/BF02982709 [DOI] [PubMed] [Google Scholar]
- 7.Harper SA, Baptista LC, Roberts LM, Wherry SJ, Boxer RS, Hildreth KL, et al. Angiotensin Converting Enzyme Inhibitors Combined with Exercise for Hypertensive Seniors (The ACES Trial): Study Protocol of a Randomized Controlled Trial. Front Med (Lausanne). 2019;6:327. doi: 10.3389/fmed.2019.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kostka J, Sikora J, Guligowska A, Kostka T. Quadriceps muscle power and optimal shortening velocity are inversely related to angiotensin converting enzyme activity in older men. F1000Res. 2021;10:184. doi: 10.12688/f1000research.51208.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Bois P, Pablo Tortola C, Lodka D, Kny M, Schmidt F, Song K, et al. Angiotensin II Induces Skeletal Muscle Atrophy by Activating TFEB-Mediated MuRF1 Expression. Circ Res. 2015;117(5):424–36. doi: 10.1161/CIRCRESAHA.114.305393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brink M, Price SR, Chrast J, Bailey JL, Anwar A, Mitch WE, et al. Angiotensin II induces skeletal muscle wasting through enhanced protein degradation and down-regulates autocrine insulin-like growth factor I. Endocrinology. 2001;142(4):1489–96. doi: 10.1210/endo.142.4.8082 [DOI] [PubMed] [Google Scholar]
- 11.Silva KAS, Ghiarone T, Schreiber K, Grant D, White T, Frisard MI, et al. Angiotensin II suppresses autophagy and disrupts ultrastructural morphology and function of mitochondria in mouse skeletal muscle. J Appl Physiol (1985). 2019;126(6):1550–62. doi: 10.1152/japplphysiol.00898.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vescovo G, Dalla Libera L, Serafini F, Leprotti C, Facchin L, Volterrani M, et al. Improved exercise tolerance after losartan and enalapril in heart failure: correlation with changes in skeletal muscle myosin heavy chain composition. Circulation. 1998;98(17):1742–9. doi: 10.1161/01.cir.98.17.1742 [DOI] [PubMed] [Google Scholar]
- 13.Bea JW, Wassertheil-Smoller S, Wertheim BC, Klimentidis Y, Chen Z, Zaslavsky O, et al. Associations between ACE-Inhibitors, Angiotensin Receptor Blockers, and Lean Body Mass in Community Dwelling Older Women. J Aging Res. 2018;2018:8491092. doi: 10.1155/2018/8491092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng TP, Nguyen TN, Gao Q, Nyunt MSZ, Wee S-L. Angiotensin receptor blockers use and changes in frailty, muscle mass, and function indexes: Singapore Longitudinal Ageing Study. JCSM rapid communications. 2021;4:111–21. [Google Scholar]
- 15.Kon SS, Jolley CJ, Shrikrishna D, Montgomery HE, Skipworth JR, Puthucheary Z, et al. ACE and response to pulmonary rehabilitation in COPD: two observational studies. BMJ Open Respir Res. 2017;4(1):e000165. doi: 10.1136/bmjresp-2016-000165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curtis KJ, Meyrick VM, Mehta B, Haji GS, Li K, Montgomery H, et al. Angiotensin-Converting Enzyme Inhibition as an Adjunct to Pulmonary Rehabilitation in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2016;194(11):1349–57. doi: 10.1164/rccm.201601-0094OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mile M, Balogh L, Papp G, Pucsok JM, Szabo K, Barna L, et al. Effects of Functional Training on Sarcopenia in Elderly Women in the Presence or Absence of ACE Inhibitors. Int J Environ Res Public Health. 2021;18(12):6594. doi: 10.3390/ijerph18126594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sumukadas D, Witham MD, Struthers AD, McMurdo ME. Effect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trial. CMAJ. 2007;177(8):867–74. doi: 10.1503/cmaj.061339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunout D, Barrera G, de la Maza MP, Leiva L, Backhouse C, Hirsch S. Effects of enalapril or nifedipine on muscle strength or functional capacity in elderly subjects. A double blind trial. J Renin Angiotensin Aldosterone Syst. 2009;10(2):77–84. doi: 10.1177/1470320309105338 [DOI] [PubMed] [Google Scholar]
- 20.Achison M, Adamson S, Akpan A, Aspray T, Avenell A, Band MM, et al. Effect of perindopril or leucine on physical performance in older people with sarcopenia: the LACE randomized controlled trial. J Cachexia Sarcopenia Muscle. 2022;13:858–71. doi: 10.1002/jcsm.12934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witham MD, Achison M, Aspray TJ, Avenell A, Band MM, Donnan PT, et al. Recruitment strategies for sarcopenia trials: lessons from the LACE randomized controlled trial. JCSM rapid communications. 2021;4(2):93–102. [Google Scholar]
- 22.Banhegyi V, Enyedi A, Fulop GA, Olah A, Siket IM, Varadi C, et al. Human Tissue Angiotensin Converting Enzyme (ACE) Activity Is Regulated by Genetic Polymorphisms, Posttranslational Modifications, Endogenous Inhibitors and Secretion in the Serum, Lungs and Heart. Cells. 2021;10(7):1708. doi: 10.3390/cells10071708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melian Ortiz A, Laguarta-Val S, Varillas-Delgado D. Muscle Work and Its Relationship with ACE and ACTN3 Polymorphisms Are Associated with the Improvement of Explosive Strength. Genes (Basel). 2021;12(8):1177. doi: 10.3390/genes12081177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folland J, Leach B, Little T, Hawker K, Myerson S, Montgomery H, et al. Angiotensin-converting enzyme genotype affects the response of human skeletal muscle to functional overload. Exp Physiol. 2000;85(5):575–9. [PubMed] [Google Scholar]
- 25.Hopkinson NS, Nickol AH, Payne J, Hawe E, Man WD, Moxham J, et al. Angiotensin converting enzyme genotype and strength in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(4):395–9. doi: 10.1164/rccm.200304-578OC [DOI] [PubMed] [Google Scholar]
- 26.Ipekoglu G, Bulbul A, Cakir HI. A meta-analysis on the association of ACE and PPARA gene variants and endurance athletic status. J Sports Med Phys Fitness. 2022;62(6):795–802. doi: 10.23736/S0022-4707.21.12417-X [DOI] [PubMed] [Google Scholar]
- 27.McAuley ABT, Hughes DC, Tsaprouni LG, Varley I, Suraci B, Roos TR, et al. The association of the ACTN3 R577X and ACE I/D polymorphisms with athlete status in football: a systematic review and meta-analysis. J Sports Sci. 2021;39(2):200–11. doi: 10.1080/02640414.2020.1812195 [DOI] [PubMed] [Google Scholar]
- 28.Kim CH, Cho JY, Jeon JY, Koh YG, Kim YM, Kim HJ, et al. ACE DD genotype is unfavorable to Korean short-term muscle power athletes. Int J Sports Med. 2010;31(1):65–71. doi: 10.1055/s-0029-1239523 [DOI] [PubMed] [Google Scholar]
- 29.Kang HJ, Kim CH, Park DS, Choi SY, Lee DH, Nam HS, et al. The Impacts of ACE Activity according to ACE I/D Polymorphisms on Muscular Functions of People Aged 65. Ann Rehabil Med. 2012;36(4):433–46. doi: 10.5535/arm.2012.36.4.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Handayani MDN, Sadewa AH, Farmawati A, Rochmah W. Deletion Polymorphism of Angiotensin-Converting Enzyme Gene Is Associated with Low Muscle Mass in Elderly People in Jakarta, Indonesia. Kobe J Med Sci. 2018;64(3):E119–E25. doi: 10.1155/2013/643575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garatachea N, Fiuza-Luces C, Torres-Luque G, Yvert T, Santiago C, Gomez-Gallego F, et al. Single and combined influence of ACE and ACTN3 genotypes on muscle phenotypes in octogenarians. Eur J Appl Physiol. 2012;112(7):2409–20. doi: 10.1007/s00421-011-2217-4 [DOI] [PubMed] [Google Scholar]
- 32.Kritchevsky SB, Nicklas BJ, Visser M, Simonsick EM, Newman AB, Harris TB, et al. Angiotensin-converting enzyme insertion/deletion genotype, exercise, and physical decline. JAMA. 2005;294(6):691–8. doi: 10.1001/jama.294.6.691 [DOI] [PubMed] [Google Scholar]
- 33.Shuler K, Sucic JF, Talley SA, Goldberg A. Angiotensin-Converting Enzyme Insertion/Deletion Polymorphism, Lower Extremity Strength, and Physical Performance in Older Adults. Phys Ther. 2019;99(8):998–1009. doi: 10.1093/ptj/pzz073 [DOI] [PubMed] [Google Scholar]
- 34.Romero-Blanco C, Artiga-Gonzalez MJ, Gomez-Cabello A, Vila-Maldonado S, Casajus JA, Ara I, et al. Strength and Endurance Training in Older Women in Relation to ACTN3 R577X and ACE I/D Polymorphisms. Int J Environ Res Public Health. 2020;17(4):1236. doi: 10.3390/ijerph17041236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang B, Tanaka H, Shono N, Miura S, Kiyonaga A, Shindo M, et al. The I allele of the angiotensin-converting enzyme gene is associated with an increased percentage of slow-twitch type I fibers in human skeletal muscle. Clin Genet. 2003;63(2):139–44. doi: 10.1034/j.1399-0004.2003.00029.x [DOI] [PubMed] [Google Scholar]
- 36.Kumagai H, Tobina T, Ichinoseki-Sekine N, Kakigi R, Tsuzuki T, Zempo H, et al. Role of selected polymorphisms in determining muscle fiber composition in Japanese men and women. J Appl Physiol (1985). 2018;124(5):1377–84. doi: 10.1152/japplphysiol.00953.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams GR, Bamman MM. Characterization and regulation of mechanical loading-induced compensatory muscle hypertrophy. Compr Physiol. 2012;2(4):2829–70. doi: 10.1002/cphy.c110066 [DOI] [PubMed] [Google Scholar]
- 38.Vikne H, Strom V, Pripp AH, Gjovaag T. Human skeletal muscle fiber type percentage and area after reduced muscle use: A systematic review and meta-analysis. Scand J Med Sci Sports. 2020;30(8):1298–317. doi: 10.1111/sms.13675 [DOI] [PubMed] [Google Scholar]
- 39.Natanek SA, Gosker HR, Slot IG, Marsh GS, Hopkinson NS, Man WD, et al. Heterogeneity of quadriceps muscle phenotype in chronic obstructive pulmonary disease (Copd); implications for stratified medicine? Muscle Nerve. 2013;48(4):488–97. doi: 10.1002/mus.23784 [DOI] [PubMed] [Google Scholar]
- 40.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23. doi: 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Band MM, Sumukadas D, Struthers AD, Avenell A, Donnan PT, Kemp PR, et al. Leucine and ACE inhibitors as therapies for sarcopenia (LACE trial): study protocol for a randomised controlled trial. Trials. 2018;19(1):6. doi: 10.1186/s13063-017-2390-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rigat B, Hubert C, Corvol P, Soubrier F. PCR detection of the insertion/deletion polymorphism of the human angiotensin converting enzyme gene (DCP1) (dipeptidyl carboxypeptidase 1). Nucleic Acids Res. 1992;20(6):1433. doi: 10.1093/nar/20.6.1433-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kehoe PG, Russ C, McIlory S, Williams H, Holmans P, Holmes C, et al. Variation in DCP1, encoding ACE, is associated with susceptibility to Alzheimer disease. Nat Genet. 1999;21(1):71–2. doi: 10.1038/5009 [DOI] [PubMed] [Google Scholar]
- 44.Sagnella GA, Rothwell MJ, Onipinla AK, Wicks PD, Cook DG, Cappuccio FP. A population study of ethnic variations in the angiotensin-converting enzyme I/D polymorphism: relationships with gender, hypertension and impaired glucose metabolism. J Hypertens. 1999;17(5):657–64. doi: 10.1097/00004872-199917050-00009 [DOI] [PubMed] [Google Scholar]
- 45.Steeds RP, Wardle A, Smith PD, Martin D, Channer KS, Samani NJ. Analysis of the postulated interaction between the angiotensin II sub-type 1 receptor gene A1166C polymorphism and the insertion/deletion polymorphism of the angiotensin converting enzyme gene on risk of myocardial infarction. Atherosclerosis. 2001;154(1):123–8. doi: 10.1016/s0021-9150(00)00438-x [DOI] [PubMed] [Google Scholar]
- 46.Narain Y, Yip A, Murphy T, Brayne C, Easton D, Evans JG, et al. The ACE gene and Alzheimer’s disease susceptibility. J Med Genet. 2000;37(9):695–7. doi: 10.1136/jmg.37.9.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montgomery HE, Clarkson P, Dollery CM, Prasad K, Losi MA, Hemingway H, et al. Association of angiotensin-converting enzyme gene I/D polymorphism with change in left ventricular mass in response to physical training. Circulation. 1997;96(3):741–7. doi: 10.1161/01.cir.96.3.741 [DOI] [PubMed] [Google Scholar]
- 48.Kee F, Morrison C, Poirier O, McCrum E, Mallet C, Nicaud V, et al. Angiotensin II type-I receptor and ACE polymorphisms and risk of myocardial infarction in men and women. Eur J Clin Invest. 2000;30(12):1076–82. doi: 10.1046/j.1365-2362.2000.00741.x [DOI] [PubMed] [Google Scholar]
- 49.Missouris CG, Barley J, Jeffery S, Carter ND, Singer DR, MacGregor GA. Genetic risk for renal artery stenosis: association with deletion polymorphism in angiotensin 1-converting enzyme gene. Kidney Int. 1996;49(2):534–7. doi: 10.1038/ki.1996.76 [DOI] [PubMed] [Google Scholar]
- 50.Markus HS, Barley J, Lunt R, Bland JM, Jeffery S, Carter ND, et al. Angiotensin-converting enzyme gene deletion polymorphism. A new risk factor for lacunar stroke but not carotid atheroma. Stroke. 1995;26(8):1329–33. doi: 10.1161/01.str.26.8.1329 [DOI] [PubMed] [Google Scholar]
- 51.Samani NJ, O’Toole L, Martin D, Rai H, Fletcher S, Lodwick D, et al. Insertion/deletion polymorphism in the angiotensin-converting enzyme gene and risk of and prognosis after myocardial infarction. J Am Coll Cardiol. 1996;28(2):338–44. doi: 10.1016/0735-1097(96)00139-8 [DOI] [PubMed] [Google Scholar]
- 52.Keavney B, McKenzie C, Parish S, Palmer A, Clark S, Youngman L, et al. Large-scale test of hypothesised associations between the angiotensin-converting-enzyme insertion/deletion polymorphism and myocardial infarction in about 5000 cases and 6000 controls. International Studies of Infarct Survival (ISIS) Collaborators. Lancet. 2000;355(9202):434–42. doi: 10.1016/s0140-6736(00)82009-7 [DOI] [PubMed] [Google Scholar]
- 53.Barley J, Blackwood A, Sagnella G, Markandu N, MacGregor G, Carter N. Angiotensinogen Met235—>Thr polymorphism in a London normotensive and hypertensive black and white population. J Hum Hypertens. 1994;8(8):639–40. [PubMed] [Google Scholar]
- 54.Ueda S, Weir CJ, Inglis GC, Murray GD, Muir KW, Lees KR. Lack of association between angiotensin converting enzyme gene insertion/deletion polymorphism and stroke. J Hypertens. 1995;13(12 Pt 2):1597–601. [PubMed] [Google Scholar]
- 55.Catto A, Carter AM, Barrett JH, Stickland M, Bamford J, Davies JA, et al. Angiotensin-converting enzyme insertion/deletion polymorphism and cerebrovascular disease. Stroke. 1996;27(3):435–40. [PubMed] [Google Scholar]
- 56.Adamzik M, Frey U, Sixt S, Knemeyer L, Beiderlinden M, Peters J, et al. ACE I/D but not AGT (-6)A/G polymorphism is a risk factor for mortality in ARDS. Eur Respir J. 2007;29(3):482–8. doi: 10.1183/09031936.00046106 [DOI] [PubMed] [Google Scholar]
- 57.Sayed-Tabatabaei FA, Schut AF, Arias Vasquez A, Bertoli-Avella AM, Hofman A, Witteman JC, et al. Angiotensin converting enzyme gene polymorphism and cardiovascular morbidity and mortality: the Rotterdam Study. J Med Genet. 2005;42(1):26–30. doi: 10.1136/jmg.2004.022756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bleumink GS, Schut AF, Sturkenboom MC, van Duijn CM, Deckers JW, Hofman A, et al. Mortality in patients with hypertension on angiotensin-I converting enzyme (ACE)-inhibitor treatment is influenced by the ACE insertion/deletion polymorphism. Pharmacogenet Genomics. 2005;15(2):75–81. doi: 10.1097/01213011-200502000-00003 [DOI] [PubMed] [Google Scholar]
- 59.Forero DA, Pinzon J, Arboleda GH, Yunis JJ, Alvarez C, Catano N, et al. Analysis of common polymorphisms in angiotensin-converting enzyme and apolipoprotein e genes and human longevity in Colombia. Arch Med Res. 2006;37(7):890–4. doi: 10.1016/j.arcmed.2006.04.001 [DOI] [PubMed] [Google Scholar]
- 60.Fiuza-Luces C, Ruiz JR, Rodriguez-Romo G, Santiago C, Gomez-Gallego F, Cano-Nieto A, et al. Is the ACE I/D polymorphism associated with extreme longevity? A study on a Spanish cohort. J Renin Angiotensin Aldosterone Syst. 2011;12(3):202–7. doi: 10.1177/1470320310391505 [DOI] [PubMed] [Google Scholar]
- 61.Pati A, Mahto H, Padhi S, Panda AK. ACE deletion allele is associated with susceptibility to SARS-CoV-2 infection and mortality rate: An epidemiological study in the Asian population. Clin Chim Acta. 2020;510:455–8. doi: 10.1016/j.cca.2020.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vladeanu MC, Bojan IB, Bojan A, Iliescu D, Badescu MC, Badulescu OV, et al. Angiotensin-converting enzyme gene D-allele and the severity of coronary artery disease. Exp Ther Med. 2020;20(4):3407–11. doi: 10.3892/etm.2020.8978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li L, Murakami S. Artificial intelligence-assisted meta-analysis of the frequency of ACE I/D polymorphisms in centenarians and other long-lived individuals. medRxiv. 2022;doi: 10.1101/2022.12.10.22283306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharanya A, Ciano M, Withana S, Kemp PR, Polkey MI, Sathyapala SA. Sex differences in COPD-related quadriceps muscle dysfunction and fibre abnormalities. Chron Respir Dis. 2019;16:1479973119843650. doi: 10.1177/1479973119843650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haizlip KM, Harrison BC, Leinwand LA. Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology (Bethesda). 2015;30(1):30–9. doi: 10.1152/physiol.00024.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mishra JS, More AS, Gopalakrishnan K, Kumar S. Testosterone plays a permissive role in angiotensin II-induced hypertension and cardiac hypertrophy in male rats. Biol Reprod. 2019;100(1):139–48. doi: 10.1093/biolre/ioy179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baptista LC, Machado-Rodrigues AM, Verissimo MT, Martins RA. Exercise training improves functional status in hypertensive older adults under angiotensin converting enzymes inhibitors medication. Exp Gerontol. 2018;109:82–9. doi: 10.1016/j.exger.2017.06.013 [DOI] [PubMed] [Google Scholar]
- 68.Habouzit E, Richard H, Sanchez H, Koulmann N, Serrurier B, Monnet R, et al. Decreased muscle ACE activity enhances functional response to endurance training in rats, without change in muscle oxidative capacity or contractile phenotype. J Appl Physiol (1985). 2009;107(1):346–53. doi: 10.1152/japplphysiol.91443.2008 [DOI] [PubMed] [Google Scholar]
- 69.Yang Y, Banerjee A, Sun Y, Carter CS, Buford TW. Interactive Effects of Enalapril Administration and Novel HIIT Wheel-Bed Training in Aged Rats. Front Rehabil Sci. 2021;2. doi: 10.3389/fresc.2021.764686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sjuretharson T, Bejder J, Breenfeldt Andersen A, Bonne T, Kyhl K, Roin T, et al. Effect of angiotensin-converting enzyme inhibition on cardiovascular adaptation to exercise training. Physiol Rep. 2022;10(13):e15382. doi: 10.14814/phy2.15382 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(XLSX)
Data Availability Statement
The data are included in an excel sheet as supplementary material.