Figure 1.
Characterizing the in vitro and in vivo potency and breadth of two neutralizing antibodies
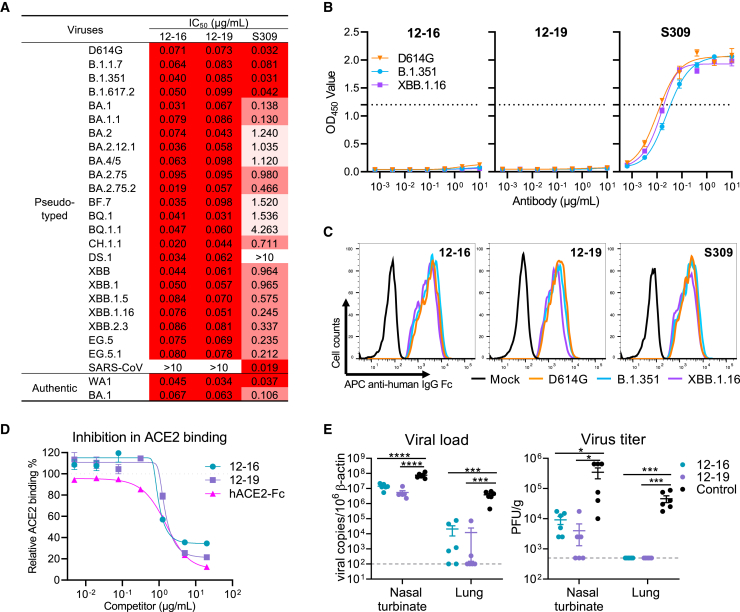
(A) Neutralization potency of 12-16 and 12-19 against pseudotyped variants and authentic viruses of SARS-CoV-2. S309 or sotrovimab was used as a control, which lost potency against Omicron subvariants.
(B) Binding assay by ELISA indicated that 12-16 and 12-19 could not bind to the SARS-CoV-2 D614G, B.1.351, and XBB.1.16 S2P spike trimers tested.
(C) Fluorescence-activated cell sorting (FACS) analysis showed that 12-16 and 12-19 well bound to the cell-surface-expressed SARS-CoV-2 D614G, B.1.351, and XBB.1.16 spike trimers, indicating that they recognize a quaternary epitope on the spike.
(D) 12-16 and 12-19 inhibited ACE2 binding to cell-surface-expressed SARS-CoV-2 D614G spike trimer. hACE2-Fc was used as a positive control.
(E) Prophylactic efficacy of 12-16 and 12-19 was evaluated in hamsters infected with Omicron variant BA.1. Viral load and titers were measured in trachea and lung 4 days post-infection. Each symbol represents an individual hamster, with a line indicating the mean of each group and error bars indicating the standard deviation. p values were determined by unpaired t test. ∗p < 0.05; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001. Dotted lines indicate assay limits of detection. Each group contained 6 animals.
Data in (A)–(D) are representative of those obtained in three independent experiments. Data in (B), (D), and (E) are presented as mean ± standard error of the mean (SEM).
See also Figures S1 and S2 and Table S1.