Figure 1.
Mural cells sustain a vascular MΦ niche
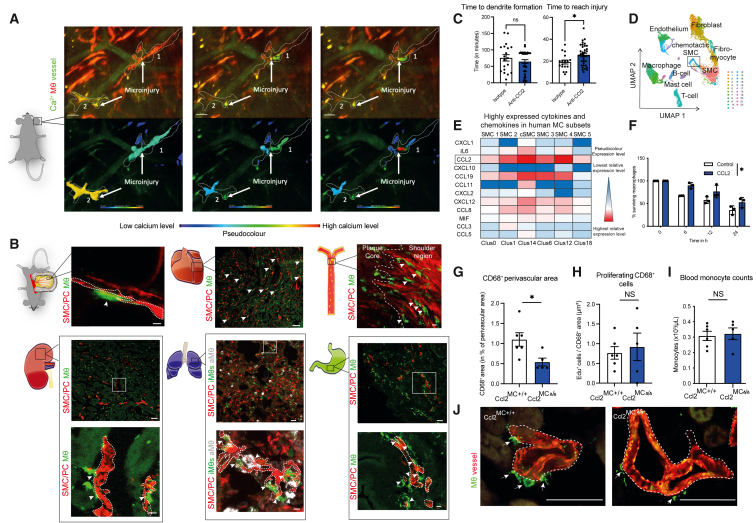
(A) In vivo multi-photon imaging of Ca2+ signal and morphological changes of MΦs in Cx3cr1-MΦCa-rep mice in an environment of laser-induced microinjuries. MΦs are depicted in red; Ca2+ signal and vascular flow are depicted in green. MΦs have been rendered additionally below, with a pseudocolored depiction of the Ca2+ signal. Images are derived from Video S1.
(B) In vivo and ex vivo confocal and airy-scan imaging of MC-MΦ contacts across organs in MCRFP-rep; Cx3cr1-MΦGFP-rep mice, arrows depicting cell-cell contacts: top left: intravital imaging of the microvasculature in the mesentery, the dashed line is depicting MCs (scale bars, 5 μm); top middle: ex vivo imaging of the heart microvasculature (scale bars, 50 μm); top right: en face ex vivo imaging of the aortic atherosclerotic intima after 3 months of western-diet feeding (macrovasculature), the dashed line is subdividing the plaque core from the shoulder region (scale bars, 10 μm); bottom left: ex vivo imaging of the kidney microvasculature (scale bars, 50 μm), including higher magnification below (dashed line depicting MCs) (scale bars, 7 μm); bottom middle: ex vivo imaging of the lung microvasculature (scale bars, 20 μm), including higher magnification below (Cx3cr1hi CD68lo interstitial MΦs (iMΦs) in green, CD68hi Cx3cr1lo alveolar MΦs (aMΦs) in white, MCs in red) (dashed line depicting MCs) (scale bars, 5 μm); bottom right: ex vivo imaging of the stomach microvasculature (scale bars, 50 μm), including higher magnification below (dashed line depicting MCs) (scale bars, 10 μm). Interstitial MΦs are shown in green, and MCs are shown in red for all organs, with further subdifferentiation of MΦs in the lung (as depicted above).
(C) Analysis of the time until MΦs form their first dendrites (left) and time which MΦs require to reach injury (right), as the time in minutes after laser injury, in MΦGFP-rep mice treated locally (subcutaneously) and systemically with isotype or CCL2-neutralizing antibody (n = 17–37 individual cells analyzed from 3 to –4 mice/group).
(D) Reanalyzed single-cell RNA-seq data from human coronary arteries from Wirka et al., GEO: GSE131780. Uniform Manifold Approximation and Projection (UMAP) based dimensionality reduction of analyzed cells.
(E) Highly expressed cytokines and chemokines in human SMCs from coronary arteries analyzed from cells shown in (D) CCL2 is highlighted as the most prominently expressed chemokine.
(F) Percentage of peritoneal macrophage survival upon CCL2 stimulation at different time points under starvation stress conditions (n = 3 experiments).
(G) Quantification of CD68+ perivascular macrophage content in Ccl2MC+/+ and Ccl2MCΔ/Δ mice in percentage of total perivascular area (15 μm radius around the vessel) in the kidney (n = 5–6 mice/group).
(H) Quantification of cell proliferation as EdU+ cells relative to CD68+ area (as number of proliferating cells/μm2).
(I) Quantification of blood monocyte counts by automated blood counter (n = 5–6).
(J) Representative images from immunofluorescence staining of kidney sections in Ccl2MCΔ/Δ and Ccl2MC+/+ mice for ACTA2 (red), CD68 (green). Scale bars, 50 μm (left: Ccl2MC+/+; right: Ccl2MCΔ/Δ). (C, G, H, and I) Student’s t test was used. (F) Repeated measures two-way ANOVA was -808990139890500used. ∗ p < 0.05. Bar graphs show mean with SEM.