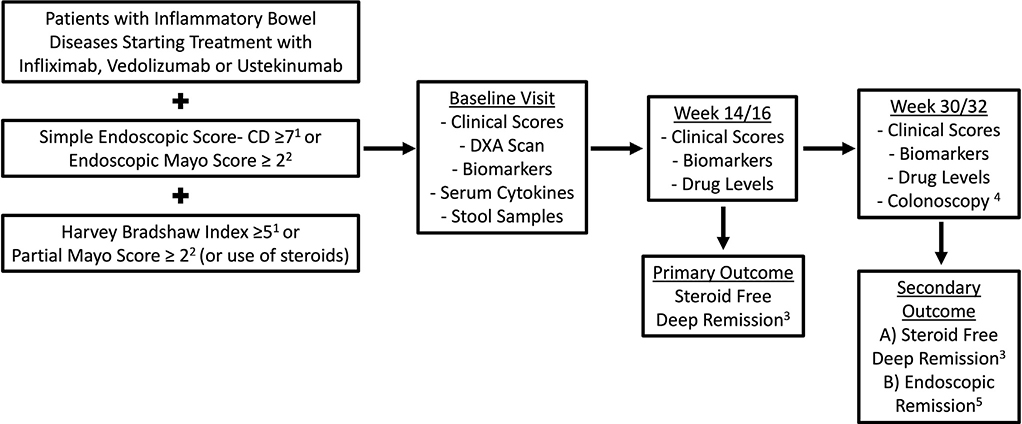
Figure 1.
Study design. 1Patients with Crohn’s disease. 2Patients with ulcerative colitis. 3Defined as a Harvey Bradshaw Index <5 or partial Mayo score <2 and normal C-reactive protein/fecal calprotectin while off steroids. 4When done between weeks 30 and 46 of therapy. 5Defined as a simple endoscopic score-CD ≤2 in Crohn’s disease and endoscopic Mayo score ≤1 in ulcerative colitis. DXA, dual-energy x-ray absorptiometry.