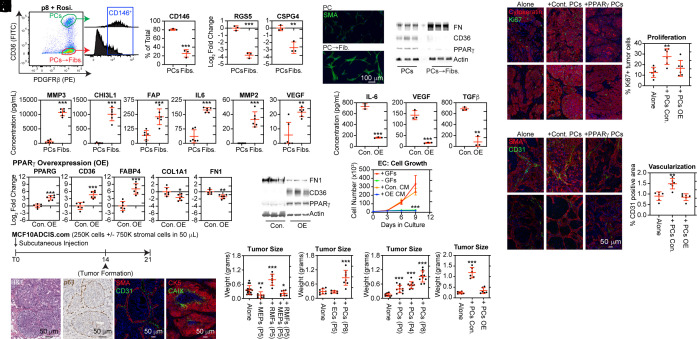
Fig. 6.
PPARγ activation suppressed protumorigenic phenotypes associated with pericyte-to-myofibroblast transition. (A) Pericytes isolated from the human breast vasculature were expanded for eight passages (P8), treated with 10 μM rosiglitazone for 24 h, and then fractionated into CD36+PDGFRβ+ pericytes (PCs) and CD36−PDGFRβ+ pericyte-derived myofibroblasts (PCsFibs.) using FACS. The CD146+ percentage was quantified. (B–D) Distinct characteristics of the populations were analyzed by (B) RT-qPCR, (C) immunocytochemistry, and (D) western blot. (E) CM collected from both populations after 24 h was analyzed by multiplex ELISA. (F and G) P4 pericytes were transduced with a lentivirus encoding human PPARγ or GFP (control) and cultured for an additional four passages (P8). PPARγ activity was validated by (F) RT-qPCR and (G) western blot. (H) CM was collected after 24 h from GFP control (Con.) and PPARγ-overexpressing (OE) pericytes and analyzed by ELISA. (I) ECs were cultured in a medium containing growth factor (+GFs), without growth factors (−GFs), with CM from control (Con. CM) or PPARγ-OE (OE CM) pericytes. (J) MCF10ADCIS.com tumors were characterized using H&E staining, IHC for p63, and mIHC for SMA and CD31 and for cytokeratin 5 (CK5) and Carbonic anhydrase IX (CAIX). (K–N) MCF10ADCIS.com were injected in mice by themselves (Alone) or coimplanted with a threefold excess of the indicated cell type. After 3 wk, the tumors were excised and weighed. The following cell types were cotransplanted: (K) myoepithelial cells (MEPs), reduction mammary fibroblasts (RMF), or a combination of both MEP and RMF; (L) ECs and pericytes (PCs); (M) uncultured (P0) pericytes directly isolated from human breast tissue, P4 pericytes, or P8 pericytes. (N) GFP control pericytes (P8) or PPARγ-OE pericytes (P8). (O) The proliferative index was determined by the percentage of cytokeratin 5 + MCF10ADCIS.com cells expressing Ki67. (P) Vascularization was compared between groups by determining the percentage of the tumor area expressing CD31.