Fig. 1.
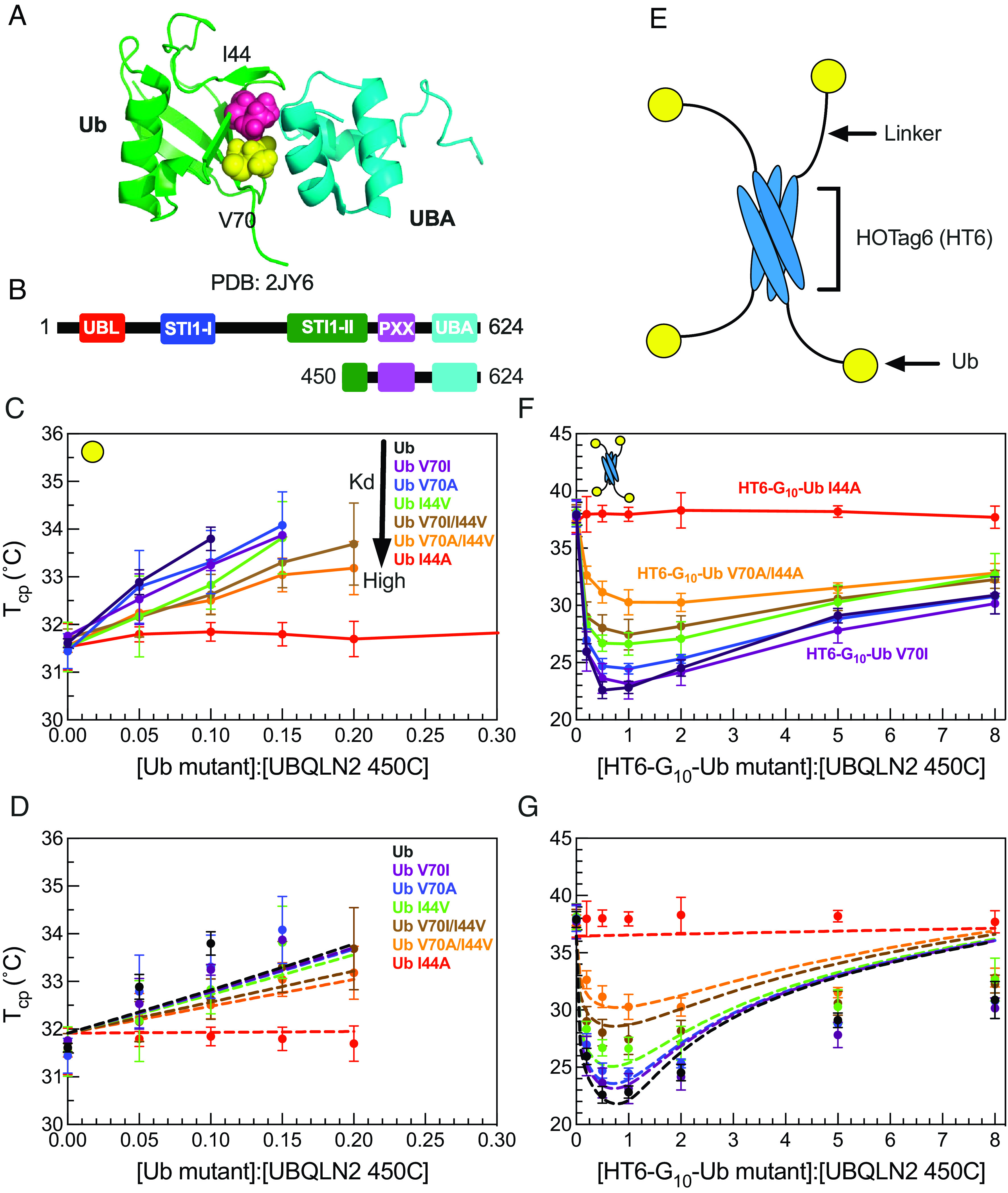
HT6-Ub variants with weakened binding affinity do not efficiently promote phase separation of UBQLN2/HT6-Ub. (A) Structure of the Ub/UBA complex with the hydrophobic patch residues I44 and V70 of Ub represented in spheres. (B) Domain structure of full-length UBQLN2 and UBQLN2 450-624 construct (450C) used in this study. (C) Cloud point temperature curves of 450C PS in the presence of monoUb variants. PS occurs above phase boundary. (D) Comparison of theory (dotted lines) to experiments (points) for the monoUb variants in panel C. (E) Architecture of HOTag6-G10-Ub (HT6-Ub) ligand. (F) Cloud point temperature curves of 450C PS in the presence of HT6-Ub variants. (G) Theory fit (dotted lines) to HT6-Ub variants using an inclusion energy of 9.5 kT. Data points and error bars in panels C and F reflect averages and SDs across at least n = 6 experiments using two protein stocks.
