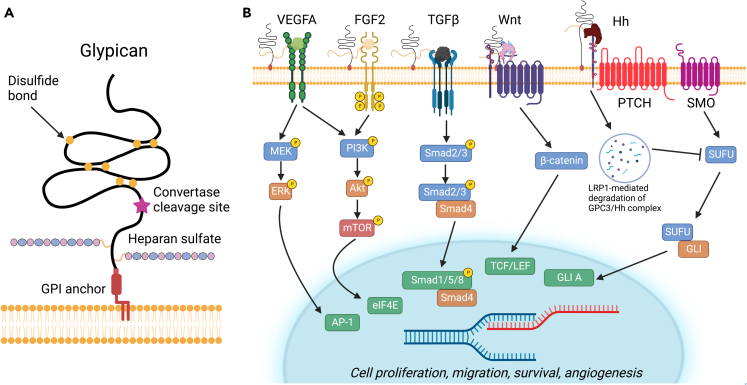
Figure 3.
Graphical overview of the structure of glypican and downstream pathway signaling effects
(A) Structure of glypican and constituent parts. Glypicans are heparan sulfate proteoglycans attached to the cell surface via a GPI lipid anchor at the C-terminus. Glypican is composed of a ∼450 kDa N-terminal protein domain containing fourteen conserved Cys residues that form seven disulfide bonds as well as a stalk region containing heparan sulfate glycosaminoglycan chains.
(B) Glypicans serve a variety of functions and affect multiple signaling cascades including the Wnt and Hedgehog pathways. In the Wnt pathway, GPC3 acts as a co-receptor and attracts Wnt to the cell surface by forming a complex with Wnt and FZD, amplifying downstream β-catenin signaling. The HS chains are not necessary for activation but rather help with stabilization of the formation of the complex. In the Hedgehog signaling pathway, GPC3 competes with PTCH for Hh binding. Mediated by LRP1, the GPC3/Hh complex undergoes endocytosis and lysosomal degradation which results in an inhibition of Hedgehog signaling. Glypicans also act as co-receptors for various angiogenic growth factors, including VEGFA, FGF2, and this interaction is thought to stabilize assembly the multi-receptor complexes. This promotes downstream signaling leading to an increase in cell proliferation and migration. GPC1 also binds TGFβ as a co-receptor and promotes SMAD signaling. For additional details, please refer to the text. Figure adapted from Pam and Ho.44