Abstract
Background and Hypothesis
Processing speed dysfunction is a core feature of psychosis and predictive of conversion in individuals at clinical high risk (CHR) for psychosis. Although traditionally measured with pen-and-paper tasks, computerized digit symbol tasks are needed to meet the increasing demand for remote assessments. Therefore we: (1) assessed the relationship between traditional and computerized processing speed measurements; (2) compared effect sizes of impairment for progressive and persistent subgroups of CHR individuals on these tasks; and (3) explored causes contributing to task performance differences.
Study Design
Participants included 92 CHR individuals and 60 healthy controls who completed clinical interviews, the Brief Assessment of Cognition in Schizophrenia Symbol Coding test, the computerized TestMyBrain Digit Symbol Matching Test, a finger-tapping task, and a self-reported motor abilities measure. Correlations, Hedges’ g, and linear models were utilized, respectively, to achieve the above aims.
Study Results
Task performance was strongly correlated (r = 0.505). A similar degree of impairment was seen between progressive (g = −0.541) and persistent (g = −0.417) groups on the paper version. The computerized task uniquely identified impairment for progressive individuals (g = −477), as the persistent group performed similarly to controls (g = −0.184). Motor abilities were related to the computerized version, but the paper version was more related to symptoms and psychosis risk level.
Conclusions
The paper symbol coding task measures impairment throughout the CHR state, while the computerized version only identifies impairment in those with worsening symptomatology. These results may be reflective of sensitivity differences, an artifact of existing subgroups, or evidence of mechanistic differences.
Keywords: processing speed, clinical high-risk for psychosis, progressive CHR syndrome, persistent CHR syndrome, cognition, computerized cognitive measure
Introduction
Processing speed, typically operationalized using digit symbol coding tasks, has been well established as the cognitive domain associated with the largest impairment for people with schizophrenia1–4 and other individuals on the psychosis spectrum.5 Recently, processing speed has become important to assess in individuals with psychosis-risk syndromes as well. In this clinical high risk for psychosis (CHR) period, cognitive impairments are attenuated compared with what is observed in schizophrenia, but processing speed remains one of the most impaired domains,6–9 particularly in individuals who later convert to a psychotic disorder.10–12 As a result, digit symbol coding is a critical component of the North American Prodrome Longitudinal Study (NAPLS) risk calculator, which estimates the vulnerability of converting to psychosis.7,13 However, in the growing landscape of remote testing, and efforts to ease research burden with measures that are easy to administer and score, there is an increased need for a computerized digit symbol measure that matches the utility of the pen-and-paper versions.
Digit symbol coding tasks are associated with many psychosis-related outcomes. Processing speed at least partially accounts for the impairment seen in all other cognitive domains in individuals at CHR,9 with first episode psychosis14 and with schizophrenia.15 In a longitudinal study examining cognition in children who later develop psychosis and their unaffected siblings, processing speed (measured by digit symbol coding) was 1 of 3 cognitive domains impaired by age 7, and the only domain that was impaired in the children who later developed psychosis compared with their unaffected siblings.16 Furthermore, processing speed is the cognitive domain most related to functional impairment in people with psychosis,17 with research indicating that it is the single best domain to predict the level of future autonomy.18 Digit symbol coding even explains some of the variance in role functioning deficits19 and predicts social functioning in individuals at risk for psychosis.20 Taken together, it is clear that processing speed measurement is crucial to understanding cognition and the prediction of psychosis.
Researchers have tried to explain why this particular domain is so impaired in people on the psychosis spectrum. For young adults without a psychiatric diagnosis, 35% of the variance of digit symbol coding performance was explained by speed tasks (half of which was graphomotor speed), 34% by visual scanning tasks, and 4%–5% by memory tasks.21 Unfortunately, a similar breakdown of the digit symbol variance explained has not been done in CHR populations. We do know that motor impairments appear in individuals who are later diagnosed with a psychotic disorder across their lifespan,22–24 including in graphomotor abilities,25–27 which could explain some of the impairment. It has been proposed that antipsychotic medication explains the impairment,28,29 but other studies either did not find an effect of2 or found improvement with4 medication. Medication also does not explain why processing speed impairment is more severe in people who later transition to a psychotic disorder.10,11 Alternatively, elevations in negative symptoms (eg, avolition) may also impact processing speed performance.30,31 Age and chronicity might also explain processing speed abilities, as impairment is worse with early onset and chronic disorders, though marked processing speed impairment remains regardless of chronicity and age of onset compared with other domains.2
Digit symbol coding is primarily assessed with a traditional pen-and-paper measure. Participants are shown a key where the numbers 1–9 are matched to unique symbols. They are then given a page full of the symbols in a random order and are asked to write in the corresponding number from the key (or the other way around). However, fueled by growing technology and efforts to reduce the research burden, ignited by the COVID-19 pandemic, and sustained by the ability to recruit larger and more representative samples, there is a need to develop comparable computerized neurocognitive measures that can be given remotely. Unfortunately, despite digit symbol coding being a core cognitive assessment of the psychosis spectrum, an equivalent computerized version has not been established. There have been several attempts to create one, dating back to 1982.32–34 One popular computerized adaptation was adopted into the Penn Computerized Neurocognitive Battery.35 People with schizophrenia are impaired on this task,35 but this task has not been directly compared with the paper version for individuals on the psychosis spectrum, nor is it validated in the CHR period. Additionally, the Brief Assessment of Cognition app has a version that is highly correlated with the paper version, but it requires special equipment and software to administer.36,37 While there has been tremendous utility in these computerized processing speed tasks, they have not yet been validated as an easily scalable replacement for the classic pen-and-paper version in the CHR population.
In response to the need to create valid computerized versions of timed, performance-based experiments, the TestMyBrain group created a novel computerized battery that is readily available for use.38 The Digit Symbol Matching Test (DSMT) from this battery is a computerized digit symbol coding task that has been validated in a very large sample including data from across the world.39,40 Another benefit to this test is that it is free to use and easily accessible, increasing its clinical utility. In this version, participants see a similar key as the paper version, but each of the 9 symbols are matched with the numbers 1, 2, or 3. Participants are shown symbols one at a time and instructed to press the number that matches it. Up to this point, however, this novel computerized assessment of processing speed has not been directly compared with the pen-and-paper version.
The Computerized Assessment of Psychosis Risk (CAPR)41 project presents a unique opportunity to directly compare the pen-and-paper symbol coding task with this computerized adaptation. Thus, the current study sought to leverage data from the CAPR project to examine 3 primary aims. (1) Assess the convergent validity of the Brief Assessment of Cognition in Schizophrenia (BACS) Symbol Coding test (referred to as BACS Symbol Coding [BSC] for brevity) and the TestMyBrain DSMT; (2) Compare these 2 tests in their ability to measure processing speed impairment in CHR individuals who are in progressive or persistent phases of their syndromes. As the BSC is typically found to be the test with the largest impairment in psychosis-spectrum disorders, we hypothesized that the BSC would measure larger impairment than the DSMT; and (3) Explore the causes of any observed differences in performance on these tasks. We hypothesize that motor ability (as examined by Finger Tapping and the Motor and Activity Psychosis-Risk Scale), attenuated positive and negative symptomatology, and psychosis risk estimates will have a greater impact on the performance of the BSC Symbol Coding test compared with the DSMT.
Methods
Participants
369 individuals aged 15–34 years old were recruited to participate in CAPR, a multisite study aimed to improve the prediction of conversion to psychosis among CHR individuals. The protocol was approved through a central Institutional Review Board process managed by Northwestern University and approved at all other sites. Participants were recruited from all over the United States by 7 participating universities: Northwestern University, University of California at Irvine, University of Maryland–Baltimore County, University of Georgia, Emory University, Yale University, and Temple University. Participants who completed any part of the study were 240 CHR individuals and 129 healthy controls (HC). However, the DSMT was introduced into the testing battery a year into data collection and data is missing on both tasks for several participants (see supplementary material). Therefore, data were available for 60 HCs and 92 CHR individuals on both tasks and thus are used for all main text analyses. Demographics are described in table 1. An additional subset of individuals completed just one of the tasks, and a replication of aims 2 and 3 with all available data can be found in supplementary material.
Table 1.
Demographics and clinical information
| HC (n=60) | CHR Persistence (n=40) | CHR Progression (n=52) | Significant Differences | |
|---|---|---|---|---|
| Demographics | ||||
| Age | 24.09 (4.25) | 24.16 (4.98) | 23.12 (4.62) | ns |
| % Female | 56.7 | 72.5 | 73.1 | ns |
| % White | 48.3 | 62.5 | 59.6 | ns |
| % Black | 16.7 | 17.5 | 26.9 | ns |
| % Asian | 38.3 | 25.0 | 21.2 | HC > Prog |
| % Indigenous | 0.0 | 7.5 | 9.6 | HC < Prog |
| Clinical Information | ||||
| SIPS P1 | 0.59 (0.75) | 2.90 (1.34) | 3.52 (1.23) | HC < Pers < Prog |
| SIPS P2 | 0.36 (0.61) | 2.48 (1.38) | 1.96 (1.50) | HC < Pers & Prog |
| SIPS P3 | 0.15 (0.45) | 0.88 (1.07) | 1.29 (1.54) | HC < Pers & Prog |
| SIPS P4 | 0.34 (0.63) | 2.17 (1.55) | 3.04 (1.08) | HC < Pers < Prog |
| SIPS P5 | 0.30 (0.59) | 1.55 (1.11) | 1.69 (1.26) | HC < Pers & Prog |
| NSI-PR Avolition | 1.00 (0.71) | 3.38 (2.08) | 3.54 (1.91) | HC < Pers & Prog |
| NSI-PR Asociality | 4.00 (1.41) | 5.22 (2.97) | 5.62 (2.73) | ns |
| NSI-PR Anhedonia | 3.25 (0.96) | 2.67 (2.50) | 2.94 (2.90) | ns |
| NSI-PR Blunted Affect | 2.00 (1.58) | 2.79 (2.87) | 4.42 (4.86) | HC < Prog |
| NSI-PR Alogia | 0.00 (0.00) | 0.56 (0.91) | 0.59 (0.80) | HC < Pers & Prog |
Participants completed clinical assessments, questionnaires, and computerized cognitive tasks across multiple virtual visits. Participants were excluded if they endorsed a lifetime intellectual disability, history of tic disorder, neurological disorder, psychotic disorder, or if they sustained a moderate to severe traumatic brain injury (rated 7 or higher on the NAPLS-2 TBI Screener42). HC were also excluded if they endorsed past or current serious psychopathology on the Structured Clinical Interview for the DSM-543 (note: past mild substance use was allowed). Participants in the CHR group met for either progressive or persistent psychosis-risk syndrome criteria (see SIPS below).
Measures
BACS Symbol Coding (BSC).44–46
Participants are shown a key where the digits 1–9 are matched with various symbols. Below, the remainder of the page is filled with the same symbols in a randomized order. Participants are instructed to match as many numbers to the symbols from the key as they can in 90 seconds going in order, without skipping any, until they are told to stop. This measure was administered remotely while being monitored by trained research staff via zoom. The primary measures used for this study are the total correct responses and total number of trials completed.
TestMyBrain Digit Symbol Matching Test (DSMT).39,40
This test was developed as a computerized version of the BSC. Similar to the BSC, participants are given a key that matches digits to 9 symbols. However, for the DSMT, the numbers 1–3 are repeated 3 times. In this test, test symbols are shown on screen one at a time until participants press the 1, 2, or 3 keys, with the intention of pressing the key that matches the symbol. Participants are instructed to match as many digits to symbols as they can in 90 seconds. Though this measure was administered remotely, trained research staff supervised participants via zoom to assure task compliance. The primary measures used for this study are the total correct responses and total number of trials completed.
Structured Interview for Psychosis-Risk Syndromes (SIPS).47
The SIPS is a semi-structured interview to assess psychosis-risk syndromes, which has shown predictive validity of conversion to psychosis, in addition to specificity and interrater reliability.48 Those diagnosed with CHR syndrome are further classified as progression or persistence. Progression indicates that symptoms started or worsened in the last year, while persistence indicates that symptoms were consistent or lessening (but still present) in the last year. SIPS interviews were administered by certified assessors who underwent extensive training led by a SIPS-certified trainer and met reliability standards. Additionally, all CHR diagnoses were verified during multisite consensus meetings.
Negative Symptom Inventory-Psychosis Risk (NSI-PR).49–51
The NSI-PR is a semi-structured interview designed to assess the presentation of negative symptoms in the CHR population. This interview generates ratings for 5 negative symptom domains: avolition, anhedonia, asociality, blunted affect, and alogia.
Wide Range Achievement Test, Reading Subtest (WRAT).52
Participants are given a list of words with increasing difficulty and irregularity and are instructed to read the words in order until they get to the end and are instructed to stop. The WRAT was used as an estimate of intelligence quotient (IQ).
Finger-tapping Speed Condition.41
This is a computerized version of the neuropsychological finger-tapping test. Participants are instructed to press a key on the keyboard as fast as they can for 30 seconds. Participants alternate hands, totaling 3 trials for each hand. The average taps in 30 seconds per dominant hand was the variable used in this study.
Sensorimotor and Activity Psychosis-Risk Scale (SMAP-R).53
This 14-item questionnaire includes questions about early developmental motor delays, the frequency of abnormal sensorimotor experiences, general assessments of sensorimotor function, and frequency of physical activity. There is an Activity scale and a Motor Abnormalities scale. The Motor Abnormalities scale can be further broken down into Coordination and Dyskinesia subscales. The Motor Abnormalities scale is of interest in this study.
Analyses
Analyses were conducted in R v4.2.2.54 For aim 1, we examined the correlation between the BSC and DSMT total scores, as well as the number of trials seen (including incorrect trials). Furthermore, we analyzed the partial correlations controlling for WRAT to examine how much of the correlation is partialed out when considering a rough IQ estimate.
For aim 2, we calculated the Hedges’ g effect sizes to assess how processing speed ability differs in persistent and progressive CHR states compared with controls on these tasks.
For aim 3, we first used linear regression to identify whether motor ability on both the MAP-R and finger tapping predicted performance on either of these tasks. Linear regressions were also used to assess whether positive and negative symptomatology predicted symbol coding ability. Each of the 5 positive symptoms assessed on the SIPS along with the total score and each of the 5 negative symptoms assessed on the NSI-PR and its total score were used to predict the total correct score on both the BSC and the DSMT. Lastly, we assessed whether the SHARP-SIPS-risk calculator55 could predict performance on either the BSC or DSMT.
Results
Descriptive statistics and distribution of scores for the BSC and DSMT primary variables can be found in supplementary table S1 and supplementary figure S1. Total correct scores on the BSC and DSMT were significantly correlated [r = 0.505, 95% CI = 0.376–0.615, P = 3.16e−11], even when controlling for WRAT IQ [rp = 0.471, 95% CI = 0.376–0.555, P = 1.51e−17], indicating a high effect size of agreement. This was similar for total trials completed [r = 0.504, 95% CI = 0.364–0.622, P = 8.34e−10; WRAT IQ controlled rp = 0.405, 95% CI = 0.305–0.497, P = 5.09e−13]. Since total correct trials are the typical primary dependent measure for digit symbol, and relationship is similar for total trials completed, the remainder of results will focus on total correct trials. Of note, the BSC had an average accuracy of 99.5% ± 1.3% while the DSMT had an average accuracy of 95.7% ± 3.7%, which is a significant difference [t(138)= 11.74, P < 2.2e−16]. Distributions of the accuracy rates can be found in supplementary figure S2 and there were no outliers on accuracy.
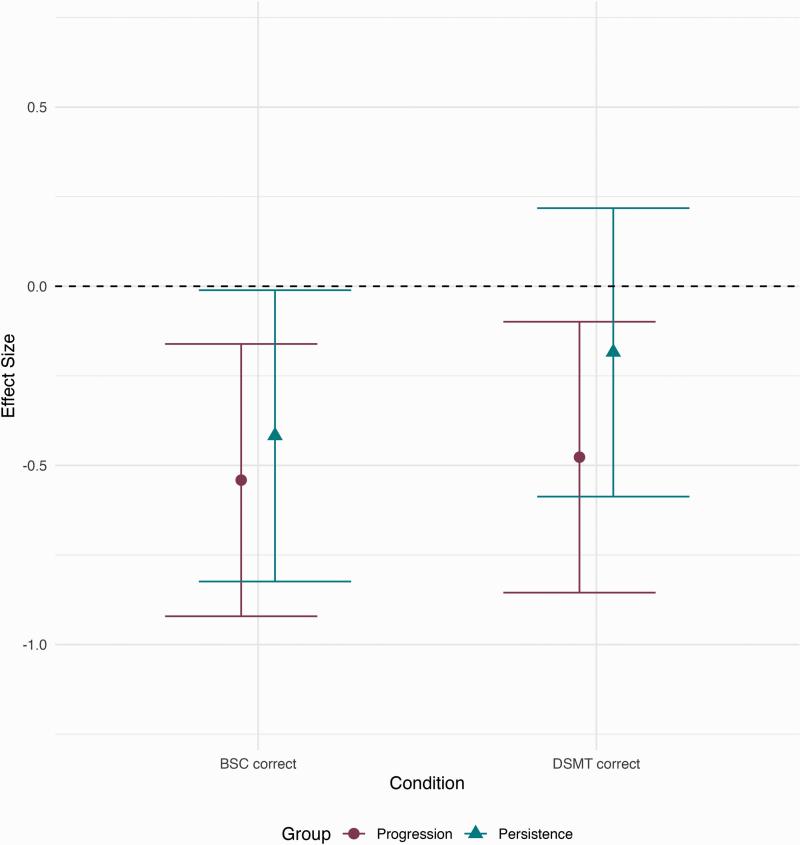
Next, we examined the effect size of processing speed ability differences in people with CHR progression and persistence on the BSC and DSMT compared with HCs (see figure 1). On the BSC, individuals whose CHR symptoms had progressed in the last year performed significantly worse compared with HCs [g = −0.540]. Those with persistent symptoms had similar impairment to progressive individuals on the BSC [g = −0.417]. Interestingly, the DSMT yielded a separation of performance between progressive and persistent CHR individuals. Similar to the BSC, individuals with a progressive CHR syndrome showed significant impairment compared with HCs [g = −0.477], while persistent individuals performed similarly to HCs [g = −0.184]. When examining the effect size of processing speed ability for progressive individuals compared with persistent, there is a negligible difference on the BSC [g = −0.116, 95% CI: −0.531 to 0.298], but there was a small effect size difference on the DSMT [g = −0.329, 95% CI: −0.746 to 0.089]. This pattern is even more pronounced in the replication that included all individuals who did just one of the tasks (figure S3).
Fig. 1.
Effect sizes of processing speed ability differences in individuals with progressive and persistent CHR syndromes relative to HCs. The black dotted line is the reference for HCs and the circles and triangles represent the effect sizes. The error bars represent 95% confidence intervals.
Since there are differences in the way the BSC and the DSMT measure processing speed ability in CHR individuals, we conducted secondary analyses to understand why. As the BSC appears to be more motorically demanding and there is established motor slowing in individuals at CHR, we hypothesized that motor ability could be driving these differences. However, finger-tapping speed did not predict BSC score [b = 0.154, t(149)=1.293, P = .198], and did predict DSMT score [b = 0.213, t(149)=2.613, P = .010]; these findings did significantly differ. However, self-reported motor functioning did not predict either the BSC [b = −0.235, t(159)= −1.257, P = .211] or DSMT [b = 0.058, t(159) = 0.434, P = .665].
We also considered that symptomatology could explain the differing performances observed on the BSC and DSMT. For attenuated positive symptoms, Persecution [b = −1.168, t(159) = −2.047, P = .042], Disorganized Communication [b = −1.628, t(159) = −2.251, P = .026], and total positive symptoms [b = −0.326, t(158) = −2.022, P = .045] negatively related to BSC total score, while DSMT negatively related to Unusual Ideas [b = −0.756, t(159) = −2.103, P = .037], Disorganized Communication [b = −1.091, t(159) = −2.127, P = .035], and total positive symptoms [b = −0.226, t(158) = −1.982, P = .049]. For negative symptoms, asociality [b = −1.220, t(102) = −3.465, P = .0008] and total negative symptoms [b = −0.282, t(98) = −2.307, P = .023] negatively related to BSC score. Asociality also negatively related to DSMT score [b = −0.566, t(102) = −2.208, P = .030], which was significantly less strong than the relationship with the BSC. The BSC was negatively related to the SHARP group’s SIPS-Risk Calculator score [b = −1.52, t(102) = −2.338, P = .0213], whereas the DSMT was not [b = −0.646, t(102) = −1.797, P = .075], which was a significant difference. However, the DSMT total score was related to the NAPLS Risk Calculator [1 year: b = −0.424, t(144) = −2.859, P = .005; 2 years: b = −0.329, t(144) = −2.885, P = .005]. The relationship between the BSC and the NAPLS Risk Calculator was not assessed as the BSC score is part of their risk calculation. These relationships were mostly replicated in the larger sample, although the relationship to positive symptoms was stronger for the BSC (see supplementary material).
Discussion
In this study, we compared a classic pen-and-paper symbol coding subtest from the BACS to a novel computer adaptation from the TestMyBrain team. With remote assessments increasing in frequency, it is imperative to have a computerized processing speed task alternative, as this domain is particularly impaired in psychosis-spectrum disorders. The two digit symbol tests were strongly correlated, even when accounting for IQ. When examining how individuals at CHR differed from HC in their processing speed ability, those with a progressive CHR syndrome had a significant impairment on the BSC which was marginally more severe than those with a persistent syndrome on the BSC. This impairment was similar for the progressive CHR subtype on the DSMT, but persistent individuals performed more similarly to controls on this task. Though the BSC captured the hypothesized symbol coding impairment better than the DSMT, the DSMT was better at differentiating progressive from persistent CHR individuals.
We first examined whether individuals performed similarly on the BSC and DSMT. The two symbol coding tasks were strongly positively correlated, both in terms of the standard measurement of total correct trials and the total number of trials completed. This relationship remained true even when considering the effect of estimated IQ on processing speed abilities. Thus, individuals who performed well on the classic pen-and-paper version of the task generally did well on the novel computerized version. Notably, this relationship is not as strong as what has been observed with the Brief Assessment of Cognition (BAC) app and remote version of the same measure, which has a correlation of about 0.8 with the BSC in 3 different populations (not tested in CHR populations).36,37 In the BAC app version, participants enter numbers on a digital keypad below the prompted symbol. It may be that the symbols each have a unique number associated with them, like the BSC, which might foster a stronger association between the symbols and the numbers. However, currently, the BAC app version requires special hardware (iPads) and software, and therefore the TestMyBrain version may be an acceptable replacement for widespread remote administration when those specialized conditions are not possible.
We then assessed how CHR individuals who had either progressing symptoms or persistent symptoms performed compared with HCs on these tasks. Individuals with a progressive CHR syndrome were impaired by an effect size of about 0.5 HCs on both tasks. This finding is somewhat attenuated, though in agreement, with previous literature examining processing speed abilities in CHR individuals.6,10 However, performance on these tasks diverged when examining individuals with a persistent CHR syndrome. On the BSC, persistent individuals performed only marginally better than progressive individuals, whereas on the DSMT, persistent individuals were only negligibly impaired compared with HCs. This was surprising given the knowledge that the BSC differentiates converters from nonconverters10,11 and that it is among the tasks that best differentiates people who remit in their CHR syndrome from nonremitters.56
There is little extant research separating progressive and persistent individuals, and it is notable that differences between these groups would be observed using one processing speed measurement, but not another. To attempt to explain this anomaly, we assessed how performance on these two tasks related to motor ability, current attenuated positive and negative symptoms, and overall psychosis vulnerability. Against out hypothesis, finger-tapping speed was positively related to the DSMT, but not the BSC. It is possible that this relationship may be driven by the fact that the finger-tapping task and DSMT both involve key presses as their motor movements, whereas the BSC requires graphomotor movements. Though there is limited research on finger-tapping deficits in CHR individuals, one study found significant motor slowing compared with HC on a finger-tapping task,57 although differences between those with progressive and persistent syndromes has not been examined. Previous literature supports that significant motor abnormalities are present in the CHR period27,53,57–60 and that they are predictive of conversion to a psychotic disorder.23,24,61,62 However, self-reported motor symptoms did not relate to performance on either the BSC or DSMT, and does not explain the performance deficit on either task or the progression/persistence divide on the DSMT.
Positive symptoms were weakly related to both tasks (though this relationship was stronger for the BSC in supplementary material replication), but negative symptoms related significantly more strongly with the BSC than the DSMT. There is no literature establishing a relationship between attenuated positive symptoms and digit symbol coding behavior, but there is evidence that negative symptoms might worsen performance.30,31 In our sample, negative symptoms do not differ significantly between persistent and progressive individuals. Therefore, the influence that negative symptoms have on the BSC may partially explain why both CHR subgroups performed similarly. Additionally, BSC scores were related to the SHARP group’s SIPS-Risk Calculator, which is calculated from the change in functioning and a subset of attenuated positive, negative, and general psychopathy symptoms, but the DSMT was not. This adds complexity; while the DSMT is associated with progression, it does not align with risk for conversion scores using this model. However, this finding is not inherently paradoxical, as there is no research examining conversion rates in progression versus persistent individuals. Interestingly, the DSMT did relate to the NAPLS risk calculator, which is calculated based on positive symptoms, age, BSC, Hopkins Verbal Learning Test, trauma, functioning, and familial history. Some of this relationship may be explained by the relationship with the BSC, but as the DSMT’s relationship with the NAPLS risk calculator is stronger than its relationship with the BSC, some of the other variables in the NAPLS risk calculator may provide important clues about the progression/persistence divide observed in this study. Overall, both tasks did relate to psychosis risk and the nature of these associations may yield novel insights and questions regarding processing speed mechanisms.
Our results provide important information about psychosis and psychosis risk. First, we add to the growing evidence that an impairment in processing speed is present before the onset of schizophrenia and other psychotic disorders. Second, this processing speed deficit may be more severe in people with worsening symptoms and a recent progression toward the psychosis threshold. Third, there may be a computerized digit symbol option that can be easily disseminated in remote studies without the need for specialized hardware or software. Additionally, this computerized task may also have utility for parsing out individuals with progressing symptoms and potentially even the prediction of schizophrenia and other psychotic disorders.
This study has several limitations. There is a relatively modest sample size per group and a larger sample might provide further insights into the differences that were observed between the BSC and the DSMT. Additionally, the DSMT was completed on personal devices, and according to https://testmybrain.org/RDOC_Report/, device latency might affect scores and therefore any added variance could have created noise that may have impacted our findings. However, the TestMyBrain teams reports that this is more of a concern for nonlaptop and desktop devices, which were not allowed in the present study. Furthermore, the study was limited by the other assessments that were available. Other measures (ie, graphomotor, oculomotor, executive functioning, etc.) might explain performance on one or both of these tasks better. Future studies should also include individuals who converted to a psychotic disorder in order to clarify whether the progression vs persistent finding on the DSMT infers an increased likelihood to convert to a psychotic disorder.
This is the first study to identify differences in persistent and progressive CHR syndromes. It remains unclear why the DSMT measures processing speed impairment in the progression CHR subtype, but not in the persistent subtype, while the BSC measures impairment in both subtypes. One likely possibility is that these results reflect sensitivity differences, with the BSC having more sensitivity to detect processing speed impairment, while the DSMT is only sensitive enough to capture more severe impairment. Another explanation could be that the DSMT is revealing the effect of true CHR subgroups. As of now, there is no evidence that psychosis conversion rates differ between persistent and progressive CHR individuals, so the DSMT might be separating CHR individuals into innate subgroups based on something other than psychosis risk. Lastly, it may be possible that performance on the BSC is driven by mechanisms core to psychosis vulnerability and symptomatology, whereas the DSMT might be specifically sensitive to mechanisms compelling worsening positive symptom psychopathology. Despite the benefits of remote testing (less training and scoring time, recruitment of larger samples, precise measurement, potential for computational modeling, etc.) and the need for a computerized assessment of processing speed, more research is needed before a recommendation can be made about whether the DSMT is an adequate alternative to the BSC to assess processing speed abilities in CHR individuals. Furthermore, it is possible that there could be different uses for the two tests depending on the specific research question. However, since individuals perform similarly on both tasks and both identify impairment, it is best to use whichever processing speed task is available for implementation in your study.
Supplementary Material
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Contributor Information
Danielle N Pratt, Department of Psychology, Northwestern University, Evanston, IL, USA.
Lauren Luther, Department of Psychology, University of Georgia, Athens, GA, USA.
Kyle S Kinney, Department of Psychology & Neuroscience, Temple University, Philadelphia, PA, USA.
Kenneth Juston Osborne, Department of Psychology, Northwestern University, Evanston, IL, USA.
Philip R Corlett, Department of Psychiatry, Yale University, New Haven, CT, USA.
Albert R Powers, III, Department of Psychiatry, Yale University, New Haven, CT, USA.
Scott W Woods, Department of Psychiatry, Yale University, New Haven, CT, USA.
James M Gold, Department of Psychiatry, Maryland Psychiatric Research Center, University of Maryland School of Medicine, Baltimore, MD, USA.
Jason Schiffman, Department of Psychological Science, University of California, Irvine, Irvine, CA, USA.
Lauren M Ellman, Department of Psychology & Neuroscience, Temple University, Philadelphia, PA, USA.
Gregory P Strauss, Department of Psychology, University of Georgia, Athens, GA, USA; Department of Neuroscience, University of Georgia, Athens, GA, USA.
Elaine F Walker, Department of Psychology and Program in Neuroscience, Emory University, Atlanta, GA, USA.
Richard Zinbarg, Department of Psychology, Northwestern University, Evanston, IL, USA.
James A Waltz, Department of Psychiatry, Maryland Psychiatric Research Center, University of Maryland School of Medicine, Baltimore, MD, USA.
Steven M Silverstein, Departments of Psychiatry, Neuroscience and Ophthalmology, University of Rochester Medical Center, Rochester, NY, USA.
Vijay A Mittal, Department of Psychology, Northwestern University, Evanston, IL, USA; Institutes for Policy Research (IPR) and Innovations in Developmental Sciences (DevSci), Psychiatry, Medical Social Sciences, Northwestern University, Evanston, IL, USA.
Funding
This work was supported by the following National Institutes of Health grants: R01 MH120090 (J.M.G.), R01 MH112613 (L.M.E.), R01 MH120091 (L.M.E.), R01 MH120092 (G.P.S.), R01 MH116039 (G.P.S./V.A.M.), R21 MH119438 (G.P.S.), R01 MH112545 (V.A.M.), R01 MH1120088 (V.A.M.), U01 MH081988 (E.F.W.), R01 MH120090 (J.A.W.), R01 MH112612 (J.S.), and R01 MH120089 (P.R.C./S.W.W.).
References
- 1.Schaefer J, Giangrande E, Weinberger DR, Dickinson D.. The global cognitive impairment in schizophrenia: consistent over decades and around the world. Schizophr Res. 2013;150(1):42–50. doi: 10.1016/j.schres.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dickinson D, Ramsey ME, Gold JM.. Overlooking the Obvious: a Meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64(5):532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- 3.Dickinson D, Ragland JD, Gold JM, Gur RC.. General and specific cognitive deficits in schizophrenia: goliath defeats David? Biol Psychiatry. 2008;64(9):823–827. doi: 10.1016/j.biopsych.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seitz-Holland J, Wojcik JD, Cetin-Karayumak S, et al. Cognitive deficits, clinical variables, and white matter microstructure in schizophrenia: a multisite harmonization study. Mol Psychiatry. 2022;27(9):3719–3730. doi: 10.1038/s41380-022-01731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.González-Blanch C, Pérez-Iglesias R, Rodríguez-Sánchez JM, et al. A digit symbol coding task as a screening instrument for cognitive impairment in first-episode psychosis. Arch Clin Neuropsychol. 2011;26(1):48–58. doi: 10.1093/arclin/acq086. [DOI] [PubMed] [Google Scholar]
- 6.Catalan A, Salazar de Pablo G, Aymerich C, et al. Neurocognitive functioning in individuals at clinical high risk for psychosis: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78:859. doi: 10.1001/jamapsychiatry.2021.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seidman LJ, Shapiro DI, Stone WS, et al. Association of neurocognition with transition to psychosis: baseline functioning in the second phase of the North American prodrome longitudinal study. JAMA Psychiatry. 2016;73(12):1239–1248. doi: 10.1001/jamapsychiatry.2016.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui H, Giuliano AJ, Zhang T, et al. Cognitive dysfunction in a psychotropic medication-naïve, clinical high-risk sample from the ShangHai-At-Risk-for-Psychosis (SHARP) study: associations with clinical outcomes. Schizophr Res. 2020;226:138–146. doi: 10.1016/j.schres.2020.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Randers L, Jepsen JRM, Fagerlund B, et al. Generalized neurocognitive impairment in individuals at ultra-high risk for psychosis: the possible key role of slowed processing speed. Brain Behav. 2021;11(3):e01962. doi: 10.1002/brb3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Millman ZB, Roemer C, Vargas T, Schiffman J, Mittal VA, Gold JM.. Neuropsychological performance among individuals at clinical high-risk for psychosis vs putatively low-risk peers with other psychopathology: a systematic review and meta-analysis. Schizophr Bull. 2022;48(5):999–1010. doi: 10.1093/schbul/sbac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hedges EP, See C, Si S, McGuire P, Dickson H, Kempton MJ.. Meta-analysis of longitudinal neurocognitive performance in people at clinical high-risk for psychosis. Psychol Med. 2022;52(11):2009–2016. doi: 10.1017/S0033291722001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tor J, Baeza I, Sintes-Estevez A, et al. Cognitive predictors of transition and remission of psychosis risk syndrome in a child and adolescent sample: longitudinal findings from the CAPRIS study. Eur Child Adolesc Psychiatry. 2023:1–16. doi: 10.1007/s00787-022-02137-w. [DOI] [PubMed] [Google Scholar]
- 13.Cannon TD, Yu C, Addington J, et al. An individualized risk calculator for research in prodromal psychosis. Am J Psychiatry. 2016;173(10):980–988. doi: 10.1176/appi.ajp.2016.15070890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodríguez-Sánchez JM, Crespo-Facorro B, González-Blanch C, Pérez-Iglesias R, Vázquez-Barquero JL, Study PG.. Cognitive dysfunction in first-episode psychosis: the processing speed hypothesis. Br J Psychiatry. 2007;191(S51):s107–s110. doi: 10.1192/bjp.191.51.s107. [DOI] [PubMed] [Google Scholar]
- 15.Ojeda N, Peña J, Schretlen DJ, et al. Hierarchical structure of the cognitive processes in schizophrenia: the fundamental role of processing speed. Schizophr Res. 2012;135(1):72–78. doi: 10.1016/j.schres.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Niendam TA, Bearden CE, Rosso IM, et al. A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. AJP. 2003;160(11):2060–2062. doi: 10.1176/appi.ajp.160.11.2060. [DOI] [PubMed] [Google Scholar]
- 17.Abella M, Vila-Badia R, Serra-Arumí C, et al. The relevance of processing speed in the functioning of people with first-episode psychosis. J Psychiatr Res. 2023;160:171–176. doi: 10.1016/j.jpsychires.2023.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Sánchez P, Ojeda N, Peña J, et al. Predictors of longitudinal changes in schizophrenia: the role of processing speed. J Clin Psychiatry. 2009;70(6):888–896. [DOI] [PubMed] [Google Scholar]
- 19.Haining K, Matrunola C, Mitchell L, et al. Neuropsychological deficits in participants at clinical high-risk for psychosis recruited from the community: relationships to functioning and clinical symptoms. Psychol Med. 2020;50(1):77–85. doi: 10.1017/S0033291718003975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrión RE, McLaughlin D, Goldberg TE, et al. Prediction of functional outcome in individuals at clinical high risk for psychosis. JAMA Psychiatry. 2013;70(11):1133–1142. doi: 10.1001/jamapsychiatry.2013.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joy S, Fein D, Kaplan E.. Decoding digit symbol: speed, memory, and visual scanning. Assessment. 2003;10(1):56–65. [DOI] [PubMed] [Google Scholar]
- 22.Walker EF, Savoie T, Davis D.. Neuromotor precursors of schizophrenia. Schizophr Bull. 1994;20(3):441–451. doi: 10.1093/schbul/20.3.441. [DOI] [PubMed] [Google Scholar]
- 23.Mittal VA, Walker EF.. Movement abnormalities predict conversion to axis I psychosis among prodromal adolescents. J Abnorm Psychol. 2007;116(4):796–803. doi: 10.1037/0021-843X.116.4.796. [DOI] [PubMed] [Google Scholar]
- 24.Dean DJ, Walther S, Bernard JA, Mittal VA.. Motor clusters reveal differences in risk for psychosis, cognitive functioning, and thalamocortical connectivity: evidence for vulnerability subtypes. Clin Psychol Sci. 2018;6(5):721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dean DJ, Teulings HL, Caligiuri M, Mittal VA.. Handwriting analysis indicates spontaneous dyskinesias in neuroleptic naïve adolescents at high risk for psychosis. J Vis Exp. 2013;(81):e50852. doi: 10.3791/50852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dean DJ, Orr JM, Newberry RE, Mittal VA.. Motor behavior reflects reduced hemispheric asymmetry in the psychosis risk period. Schizophr Res. 2016;170(1):137–142. doi: 10.1016/j.schres.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dean DJ, Bernard JA, Damme KSF, O’Reilly R, Orr JM, Mittal VA.. Longitudinal assessment and functional neuroimaging of movement variability reveal novel insights into motor dysfunction in clinical high risk for psychosis. Schizophr Bull. 2020;46(6):1567–1576. doi: 10.1093/schbul/sbaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knowles EEM, David AS, Reichenberg A.. Processing speed deficits in schizophrenia: reexamining the evidence. Am J Psychiatry. 2010;167(7):828–835. doi: 10.1176/appi.ajp.2010.09070937. [DOI] [PubMed] [Google Scholar]
- 29.Fatouros-Bergman H, Cervenka S, Flyckt L, Edman G, Farde L.. Meta-analysis of cognitive performance in drug-naïve patients with schizophrenia. Schizophr Res. 2014;158(1):156–162. doi: 10.1016/j.schres.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 30.Fervaha G, Zakzanis KK, Foussias G, Graff-Guerrero A, Agid O, Remington G.. Motivational deficits and cognitive test performance in schizophrenia. JAMA Psychiatry. 2014;71(9):1058–1065. doi: 10.1001/jamapsychiatry.2014.1105. [DOI] [PubMed] [Google Scholar]
- 31.Lindgren M, Manninen M, Laajasalo T, et al. The relationship between psychotic-like symptoms and neurocognitive performance in a general adolescent psychiatric sample. Schizophr Res. 2010;123(1):77–85. doi: 10.1016/j.schres.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 32.McLeod DR, Griffiths RR, Bigelow GE, Yingling J.. An automated version of the digit symbol substitution test (DSST). Behav Res Meth Instrum. 1982;14(5):463–466. doi: 10.3758/BF03203313. [DOI] [Google Scholar]
- 33.Chen X, Hu N, Wang Y, Gao X.. Validation of a brain-computer interface version of the digit symbol substitution test in healthy subjects. Comput Biol Med. 2020;120:103729. doi: 10.1016/j.compbiomed.2020.103729. [DOI] [PubMed] [Google Scholar]
- 34.Moore TM, Basner M, Nasrini J, et al. Validation of the cognition test battery for spaceflight in a sample of highly educated adults. Aerosp Med Hum Perform. 2017;88(10):937–946. doi: 10.3357/AMHP.4801.2017. [DOI] [PubMed] [Google Scholar]
- 35.Bachman P, Reichenberg A, Rice P, et al. Deconstructing processing speed deficits in schizophrenia: application of a parametric digit symbol coding test. Schizophr Res. 2010;118(1):6–11. doi: 10.1016/j.schres.2010.02.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atkins AS, Kraus MS, Welch M, et al. Remote self-administration of digital cognitive tests using the brief assessment of cognition: feasibility, reliability, and sensitivity to subjective cognitive decline. Front Psychiatry. 2022;13:910896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atkins AS, Tseng T, Vaughan A, et al. Validation of the tablet-administered Brief Assessment of Cognition (BAC App). Schizophr Res. 2017;181:100–106. doi: 10.1016/j.schres.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Germine L, Nakayama K, Duchaine BC, Chabris CF, Chatterjee G, Wilmer JB.. Is the Web as good as the lab? Comparable performance from Web and lab in cognitive/perceptual experiments. Psychon Bull Rev. 2012;19(5):847–857. doi: 10.3758/s13423-012-0296-9. [DOI] [PubMed] [Google Scholar]
- 39.Hartshorne JK, Germine LT.. When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the lifespan. Psychol Sci. 2015;26(4):433–443. doi: 10.1177/0956797614567339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh S, Strong RW, Jung L, et al. The testmybrain digital neuropsychology toolkit: development and psychometric characteristics. J Clin Exp Neuropsychol. 2021;43(8):786–795. doi: 10.1080/13803395.2021.2002269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mittal VA, Ellman LM, Strauss GP, et al. Computerized assessment of psychosis risk. J Psychiatr Brain Sci. 2021;6(3):e210011. doi: 10.20900/jpbs.20210011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deighton S, Buchy L, Cadenhead KS, et al. Traumatic brain injury in individuals at clinical high risk for psychosis. Schizophr Res. 2016;174(1):77–81. doi: 10.1016/j.schres.2016.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.First MB, Williams JB, Karg RS, Spitzer RL.. Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). Arlington, VA: American Psychiatric Association; 2015:1–94. [Google Scholar]
- 44.Wechsler D. Wechsler Adult Intelligence Scale, (WAIS-III). 3rd ed., San Antonio, TX: Psychological Corporation scan oxfordjournals org; 1997. [Google Scholar]
- 45.Keefe RSE, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L.. The brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68(2–3):283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Keefe RSE, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L.. Brief Assessment of Cognition in Schizophrenia (BACS) [Database Record]. APA Psyc Tests. 2018. doi: 10.1037/t38021-000 [DOI]
- 47.McGlashan TH, Walsh BC, Woods SW, et al. Structured Interview for Psychosis-risk Syndromes. New Haven, CT: Yale School of Medicine; 2001. [Google Scholar]
- 48.Woods SW, Walsh BC, Powers AR, McGlashan TH.. Reliability, validity, epidemiology, and cultural variation of the Structured Interview for Psychosis-Risk Syndromes (SIPS) and the Scale of Psychosis-Risk Symptoms (SOPS). In: Li H, Shapiro DI, Seidman LJ, eds. Handbook of Attenuated Psychosis Syndrome Across Cultures: International Perspectives on Early Identification and Intervention. Cham: Springer International Publishing; 2019:85–113. doi: 10.1007/978-3-030-17336-4_5. [DOI] [Google Scholar]
- 49.Pelletier-Baldelli A, Strauss GP, Visser KH, Mittal VA.. Initial development and preliminary psychometric properties of the Prodromal Inventory of Negative Symptoms (PINS). Schizophr Res. 2017;189:43–49. doi: 10.1016/j.schres.2017.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strauss GP, Pelletier-Baldelli A, Visser KF, Walker EF, Mittal VA.. A review of negative symptom assessment strategies in youth at clinical high-risk for psychosis. Schizophr Res. 2020;222:104–112. doi: 10.1016/j.schres.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strauss GP, Walker EF, Pelletier-Baldelli A, et al. Development and validation of the negative symptom inventory-psychosis risk. Schizophr Bull. 2023;49:1205–1216. doi: 10.1093/schbul/sbad038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilkinson GS, Robertson GJ.. Wide range achievement test 4. J Clin Exp Neuropsychol. 1993. [Google Scholar]
- 53.Damme KSF, Schiffman J, Ellman LM, Mittal VA.. Sensorimotor and activity psychosis-risk (SMAP-R) scale: an exploration of scale structure with replication and validation. Schizophr Bull. 2021;47(2):332–343. doi: 10.1093/schbul/sbaa138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.R Core Team. R: A Language and Environment for Statistical Computing. 2022. https://www.R-project.org/. Date accessed August 11, 2022. [Google Scholar]
- 55.Zhang T, Xu L, Tang Y, et al. ; SHARP (ShangHai At Risk for Psychosis) Study Group. Prediction of psychosis in prodrome: development and validation of a simple, personalized risk calculator. Psychol Med. 2019;49(12):1990–1998. doi: 10.1017/S0033291718002738. [DOI] [PubMed] [Google Scholar]
- 56.Lam M, Lee J, Rapisarda A, et al. Longitudinal cognitive changes in young individuals at ultrahigh risk for psychosis. JAMA Psychiatry. 2018;75(9):929–939. doi: 10.1001/jamapsychiatry.2018.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Damme KSF, Osborne KJ, Gold JM, Mittal VA.. Detecting motor slowing in clinical high-risk for psychosis in a computerized finger tapping model. Eur Arch Psychiatry Clin Neurosci. 2020;270(3):393–397. doi: 10.1007/s00406-019-01059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mittal VA, Neumann C, Saczawa M, Walker EF.. Longitudinal progression of movement abnormalities in relation to psychotic symptoms in adolescents at high risk of schizophrenia. Arch Gen Psychiatry. 2008;65(2):165–171. doi: 10.1001/archgenpsychiatry.2007.23. [DOI] [PubMed] [Google Scholar]
- 59.Osborne KJ, Walther S, Shankman SA, Mittal VA.. Psychomotor slowing in Schizophrenia: implications for endophenotype and biomarker development. Biomark Neuropsychiatry. 2020;2:100016. doi: 10.1016/j.bionps.2020.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Harten PN, Walther S, Kent JS, Sponheim SR, Mittal VA.. The clinical and prognostic value of motor abnormalities in psychosis, and the importance of instrumental assessment. Neurosci Biobehav Rev. 2017;80:476–487. doi: 10.1016/j.neubiorev.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 61.Mittal VA, Walker EF, Bearden CE, et al. Markers of basal ganglia dysfunction and conversion to psychosis: neurocognitive deficits and dyskinesias in the prodromal period. Biol Psychiatry. 2010;68(1):93–99. doi: 10.1016/j.biopsych.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Callaway DA, Perkins DO, Woods SW, Liu L, Addington J.. Movement abnormalities predict transitioning to psychosis in individuals at clinical high risk for psychosis. Schizophr Res. 2014;159(2):263–266. doi: 10.1016/j.schres.2014.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.