Abstract
Objective:
To characterize Urologic Chronic Pelvic Pain Syndrome (UCPPS) pain and urinary symptom trajectories with up to 9 years of follow-up and evaluate whether initial 1-year trajectories are associated with longer-term changes.
Materials and Methods:
Data were analyzed from the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Network’s prospective observational protocols including the Epidemiology and Phenotyping Study (EPS; baseline to Year 1), EPS Extension (EXT; Years 1–5), and Symptom Patterns Study (SPS: 3-year study; Years 3–9). Adults with Interstitial Cystitis/Bladder Pain Syndrome or Chronic Prostatitis/Chronic Pelvic Pain Syndrome provided patient-reported assessments biweekly (EPS), every 4 months (EXT), or quarterly (SPS). Primary outcomes were composite pain (0–28) and urinary (0–25) severity scores. Multi-phase mixed effects models estimated outcomes over time, adjusted for baseline severity and stratified by EPS symptom trajectory.
Results:
163 participants (52% women; mean ± SD age 46.4 ± 16.1 years) completed EPS and enrolled in EXT; 67 also enrolled in SPS. Median follow-up was 4.6 years (range 1.3–9.0). After 1 year: 27.6%, 44.8% and 27.6% and 27.0%, 38.0% and 35.0% were improved, stable or worse in pain and urinary symptom severity, respectively. On average, pain and urinary symptom scores did not change further during EXT and SPS periods.
Conclusions:
Women and men with UCPPS showed remarkable stability in pain and urinary symptom severity for up to 9 years, irrespective of their initial symptom trajectory, suggesting UCPPS is a chronic condition with stable symptoms over multiple years of follow-up.
Keywords: Urologic chronic pelvic pain syndrome, interstitial cystitis, chronic prostatitis, bladder pain syndrome, MAPP Network, cohort study, extended follow-up
Introduction
Urologic Chronic Pelvic Pain Syndrome, including IC/BPS in women and men and CP/CPPS in men, is a chronic, debilitating condition associated with bladder and/or pelvic pain, and urinary frequency and urgency. UCPPS symptoms are often present for many years before diagnoses are made, and treatments are not successful in all patients.1 The natural history of UCPPS is not well understood. Some studies have observed stable symptoms in patients over 1–3 years2, 3 while others observed improvement.4, 5 Few multi-year, prospective studies have been conducted, making it difficult to counsel patients about the likely trajectory of symptoms.
During its first phase, the MAPP Research Network conducted the multicenter, longitudinal EPS, to describe 1-year UCPPS symptom patterns.6 Many participants had stable symptoms over the 1-year study, and non-urological symptoms, including widespread pain and poorer general health, were risk factors for worsening pain and urinary symptoms.7
During development of the MAPP Network’s second phase protocol, the Trans-MAPP SPS,8 EPS participants were invited to enroll in an extension study (EXT) that continued data collection after EPS and through the initiation of SPS, a 3-year longitudinal study of some of the same and additional UCPPS participants. This yielded an unprecedented opportunity to document UCPPS symptoms for up to 9 years for some participants. Our objectives were to: 1) characterize long-term trajectories of pain and urinary symptoms in UCPPS, 2) evaluate whether initial 1-year symptom trajectories are associated with longer-term changes, and 3) identify baseline factors associated with symptom profiles over extended follow-up.
Materials and Methods
Study Design and Participants
This secondary analysis includes data collected prospectively from UCPPS participants observed over a 9-year period, spanning the MAPP Network’s EPS and SPS studies and the linking EXT protocol (EPS 2009–13; EXT 2013–15; SPS 2015–19). Institutional Review Boards at each of six clinical sites and coordinating center approved all protocols, and participants signed informed consent documents.
EPS participants enrolled in EXT who completed at least one EXT assessment were included. The EPS was an observational, prospective study of UCPPS patients. Inclusion and exclusion criteria and study results have been previously described.6, 8 Briefly, adult women and men with a clinical diagnosis of IC/BPS or CP/CPPS, who reported pain of ≥1 (0–10 scale) and urinary symptoms during 3 of the previous 6 months, were eligible. Data included patient-reported assessments collected via computer at in-person baseline, 26-week, and 52-week visits, and at biweekly on-line visits. Starting in 2013, individuals who previously completed EPS and active study participants at their final visit were invited to participate in EXT. Data collection included on-line questionnaires every 4 months extending to 5 years beyond the initial EPS baseline. Because EXT was not introduced until four years after EPS was initiated, EXT data collection began anytime between 16 and 52 months after EPS enrollment.
In 2015, the EXT study ended in preparation for initiating SPS. Inclusion/exclusion criteria were intentionally similar to EPS, and participants in EPS and EXT were eligible to enroll in SPS. Data collection included similar instruments, obtained at in-person (baseline and every 6 months) and on-line (quarterly) visits.8
Measures, Outcomes and Predictors
Baseline EPS data included demographics, medical history, and examination results. UCPPS pain and urinary symptoms and related QOL6–8 were assessed using validated patient-reported measures, including the GUPI and ICSI.9, 10. The two primary outcomes were composite Pelvic Pain Severity (PPS: range 0–28) and Urinary Symptom Severity (USS: range 0–25) scores, previously created from GUPI and ICSI items and used in MAPP Network analyses.11, 12 Potential predictors of symptom trajectory were identified a priori based on previous studies, including those associated with EPS 1-year symptom change:7 age, sex, symptom duration, and baseline assessments of widespread pain (Brief Pain Inventory13), non-urologic symptoms (Complex Medical Symptom Inventory14), mental and physical health-related QOL (SF-1215), depression and anxiety (Hospital Anxiety and Depression Scale16), stress (Perceived Stress Scale17), and catastrophizing (Coping Strategies Questionnaire - Catastrophizing18). One-year PPS and USS score profiles (improved, stable, or worse) were derived by a functional clusters approach using participants’ biweekly EPS symptom reports.7, 12, 19
Statistical Analyses
Participants included in this study were compared to EPS participants not included in baseline demographics and clinical measures using chi-square tests for categorical and t-tests for continuous measures.
We examined how PPS and USS scores changed over extended follow-up (EXT and SPS) using a multiphase mixed-effects model,20, 21 consisting of repeated assessments through multiple phases over time (EPS, EXT, and SPS). The model accommodates within-participant correlation attributable to repeated measures; the nature of change within each phase (i.e., linear, log-linear, quadratic, etc.); varying amount of elapsed time between phases; and varying number of assessments within phases. Analyses were performed in participant subgroups previously identified as improved, stable, or worse during the 1-year EPS.7
Similar to previous analyses, symptom ratings at EPS week 4 visit were used as the baseline level for modeling to reduce the impact of factors related to study entry on baseline outcomes including regression to the mean.12 We contrasted amount of change within subgroups across phases (EPS, EXT, and SPS), as well as between subgroups within phases, to determine whether, per subgroup, 1-year symptom trajectories predict further changes over the subsequent phases and to quantify differences in amount of change between subgroups within each phase.
To investigate potential predictors of long-term symptom change, we examined the impact of baseline variables on average severity per phase and change over time per phase. The 1-year symptom trajectory subgroups and potential predictors were included in the model as both main effects and interaction terms with phase, and linear contrasts were used to examine all differences. A significant main effect term indicates that the measure is predictive of severity and change in severity consistently across phases. A significant interaction term indicates the predictor behaves differentially across phases. Potential covariates included predictors previously identified and site. Covariates were retained if significant (p<0.05). To interpret prediction effects for binary predictors, we produced model-based estimates of the average severity score and change in severity score per phase at each predictor level. For continuous predictors, we produced estimates at three levels of the predictor on its continuous scale: one SD below average (“low”), average (“average”), and one SD above average (“high”).
Sample sizes for EPS and SPS were based on primary aims for those studies, and a separate calculation was not performed for this analysis. Statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC). Statistical significance was defined as p<0.05.
Results
Three hundred fifty participants who completed EPS (of 424 enrolled) were invited to participate in EXT. One hundred sixty-nine enrolled, and 164 completed at least one EXT visit. One participant with inadequate data was excluded, leaving 163 (46.6% of those eligible) in this analysis. Among those in EXT, 67 participants also enrolled in SPS. The cohort was followed for a median of 4.6 years (range 1.3–9.0; Figure 1).
Figure 1.
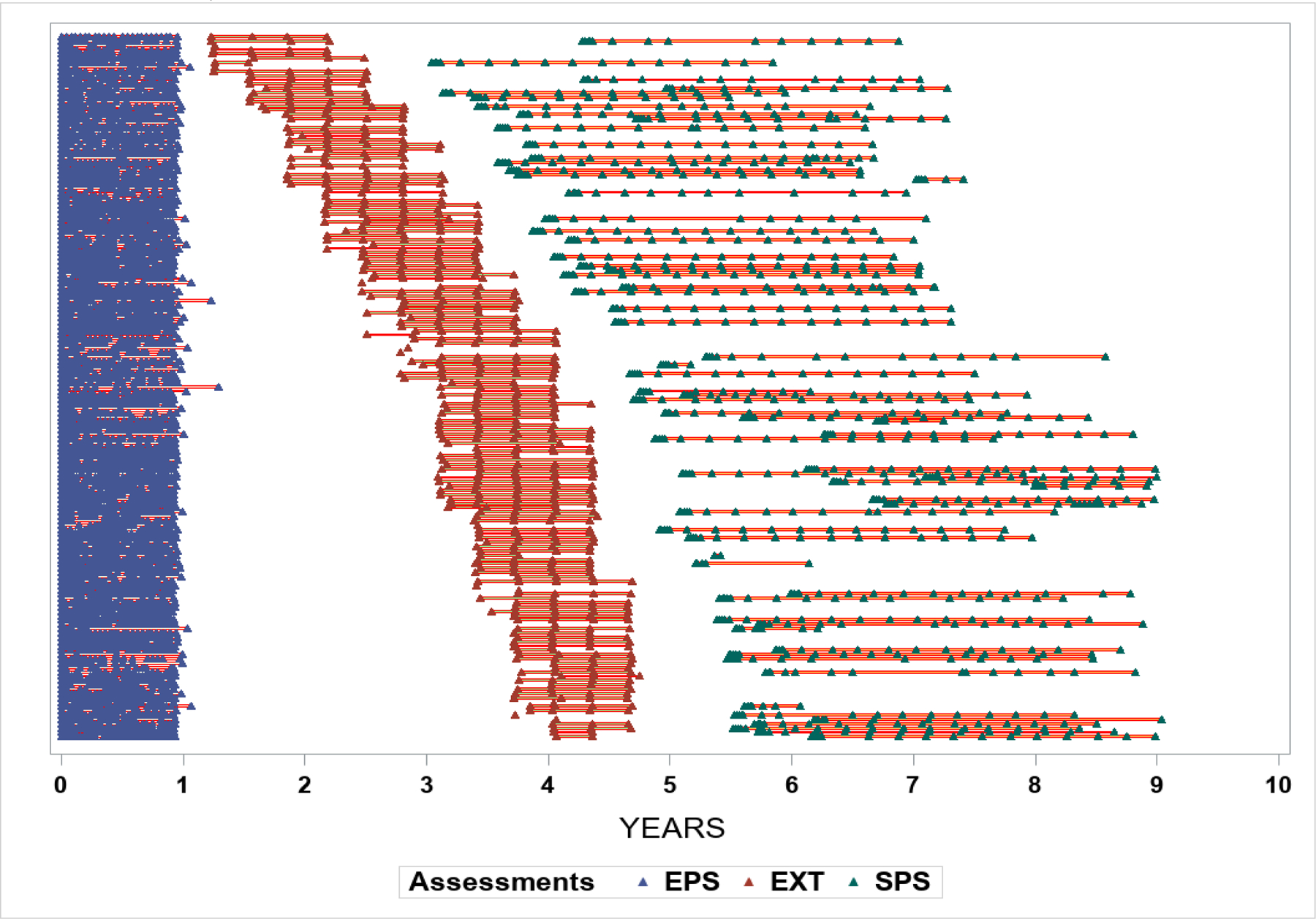
Timing of study visits for participants in the Epidemiology and Phenotyping Study (EPS), Extension (EXT) phase and Symptom Pattern Study (SPS) measured in years after the baseline EPS visit (month 0) (n=163).
The 163 participants in this study were older (46.4 vs 41.5 years, p=0.001), had greater body mass index (27.1 vs 25.6 kg/m2, p=0.006) and differed by race/ethnicity (more White (94.5% vs 88.4%, p=0.04) and less Hispanic (3.1% vs 8.8%, p=0.02)) compared to the 261 other EPS participants. Other baseline variables, including demographics, symptom duration, psychosocial outcomes, and PPS and USS scores did not differ between groups (data not shown).
During EPS, 163 participants completed 6 to 25 biweekly assessments (median 22). Participants also completed 1 to 5 EXT phase visits (90% at least 2, and 83% at least 3). Duration of EXT participation ranged from 0 to 16 months (median 12, IQR 8–12). SPS visits occurred 36 to 108 months after EPS baseline. The 67 SPS participants completed 2 to 19 (median 16, IQR 14–17) visits over 0 to 39 months (median 33, IQR 30–34).
The 163 participants’ mean age was 46.4±16.1 years. Fifty-two percent were female and 95% White. On average participants reported UCPPS symptoms for 8.9±11.0 years before EPS enrollment. At baseline, mean PPS and USS scores were 14.8±5.5 and 12.6±6.3, respectively, and 37.4% reported widespread pain (Table 1). During EPS, 45 (27.6%), 73 (44.8%) and 45 (27.6%) participants were improved, stable or worse in PPS respectively, and 44 (27.0%), 62 (38.0%) and 57 (35.0%) were improved, stable or worse in USS. Participants whose USS scores improved had higher baseline scores (mean (SD) 14.53 (5.67) vs. 11.05 (6.68) and 12.09 (5.76) for the improved, stable and worse groups, respectively; p=0.037). Other baseline characteristics did not differ by 1-year PPS or USS trajectories (Table 1).
Table 1.
Baseline participant characteristics in all participants and stratified by 1-year Pelvic Pain and Urinary Symptom trajectories
| Characteristics | All (N=163) | Pelvic Pain Severity Patterns | Urinary Symptom Severity Patterns | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Improved (n=45) |
Stable (n=73) |
Worse (n=45) |
p-value | Improved (n=44) |
Stable (N=62) |
Worse (n=57) |
p-value | ||
| Age (yrs) | 46.40 (16.07) | 47.42 (14.44) | 45.98 (17.36) | 46.04 (15.74) | 0.70 | 44.02 (15.15) | 47.65 (17.03) | 47.72 (15.85) | 0.23 |
| Gender | |||||||||
| Female | 51.5% | 53.3% | 50.7% | 51.1% | 0.83 | 49.1% | 46.8% | 61.4% | 0.27 |
| Male | 48.5% | 46.7% | 49.3% | 48.9% | 50.9% | 53.2% | 38.6% | ||
| Race | |||||||||
| White | 94.5% | 100.0% | 90.4% | 95.6% | 0.36 | 96.5% | 93.5% | 93.2% | 0.45 |
| Black | 4.3% | 0.0% | 6.8% | 4.4% | 1.8% | 4.8% | 6.8% | ||
| Other | 1.2% | 0.0% | 2.8% | 0.0% | 1.7% | 1.7% | 0.0% | ||
| Income | |||||||||
| <10K | 12.1% | 13.9% | 13.6% | 7.5% | 0.19 | 14.8% | 10.5% | 10.5% | 0.66 |
| 10–25K | 7.4% | 2.3% | 9.1% | 10.0% | 11.1% | 11.1% | 5.3% | ||
| 25–50K | 14.1% | 9.3% | 21.2% | 7.5% | 9.3% | 9.3% | 15.8% | ||
| 50–100K | 36.9% | 34.9% | 31.8% | 47.5% | 29.6% | 29.6% | 39.5% | ||
| >100K | 29.5% | 39.5% | 24.2% | 27.5% | 35.2% | 35.2% | 24.6% | ||
| Symptom duration (yrs) | 8.93 (10.97) | 9.29 (9.92) | 9.20 (11.02) | 8.11 (12.04) | 0.61 | 7.00 (10.23) | 9.33 (9.17) | 10.85 (13.76) | 0.053 |
| Body mass index (kg/m2) | 27.07 (5.59) | 26.71 (5.46) | 27.15 (6.11) | 27.29 (4.89) | 0.64 | 27.34 (6.26) | 26.74 (4.80) | 27.19 (5.84) | 0.83 |
| Any chronic overlapping pain condition | 39.3% | 40.0% | 38.4% | 40.0% | 0.99 | 42.1% | 41.9% | 31.8% | 0.33 |
| Non-pelvic pain* | |||||||||
| None | 23.3% | 26.7% | 30.1% | 8.9% | 0.08 | 26.3% | 27.4% | 13.6% | 0.48 |
| Intermediate | 39.3% | 42.2% | 35.6% | 42.2% | 40.4% | 35.5% | 43.2% | ||
| Widespread | 37.4% | 31.1% | 34.2% | 48.9% | 33.3% | 37.1% | 43.2% | ||
| Pelvic Pain Severity score | 14.76 (5.52) | 14.48 (5.59) | 15.00 (5.87) | 14.66 (4.92) | 0.88 | 15.95 (5.13) | 13.73 (6.22) | 14.69 (4.70) | 0.21 |
| Urinary Symptom Severity Score | 12.56 (6.24) | 13.02 (5.60) | 12.79 (6.98) | 11.71 (5.59) | 0.35 | 14.53 (5.67) | 11.05 (6.68) | 12.09 (5.76) | 0.037 |
| SF-12 PCS | 47.02 (10.28) | 48.65 (9.19) | 46.71 (10.96) | 45.85 (10.25) | 0.22 | 46.38 (10.57) | 48.38 (10.36) | 45.90 (9.81) | 0.94 |
| SF-12 MCS | 44.35 (10.83) | 44.73 (10.57) | 43.07 (11.17) | 46.02 (10.54) | 0.56 | 43.37 (11.65) | 44.48 (11.04) | 45.43 (9.50) | 0.36 |
| HADS Depression | 5.13 (4.30) | 4.96 (4.33) | 5.72 (4.64) | 4.34 (3.57) | 0.52 | 4.96 (4.04) | 5.56 (5.18) | 4.74 (3.13) | 0.89 |
| HADS Anxiety | 7.35 (4.45) | 7.24 (4.69) | 8.06 (4.44) | 6.33 (4.10) | 0.33 | 8.14 (4.49) | 7.15 (4.77) | 6.61 (3.83) | 0.094 |
| Perceived Stress Scale | 15.65 (8.02) | 15.58 (8.16) | 16.39 (8.18) | 14.53 (7.65) | 0.54 | 15.87 (7.43) | 15.80 (9.10) | 15.17 (7.25) | 0.70 |
p-values obtained by Mantel-Haenzel test for binary and regression for continuous measures
Data presented as % or mean (SD)
Non-pelvic pain defined by Brief Pain Inventory non-pelvic regions with pain categorized as 0 (None), 1–2 (Intermediate), 3–7 (Widespread)
HADS, Hospital Anxiety and Depression Scale; MCS, Mental Component Score; PCS, Physical Component Score
Most PPS change occurred during the first year (Figure 2A). Participants identified as “improved” reduced their pain scores by 5 points at 1-year, and this improvement was maintained (with no additional significant change) in EXT and SPS phases (average scores remained significantly improved compared to baseline (p<0.001) and not different from 1-year (p>0.05)). Pain Severity in the 1-year stable group remained remarkably stable (unchanged) throughout the remainder of follow-up (all p>0.05). Pain scores in those who worsened during EPS were still higher (worse) during EXT (2.2 points, p=0.006) and during SPS (although not statistically greater: 1.9 points, p=0.09) compared to baseline.
Figure 2.
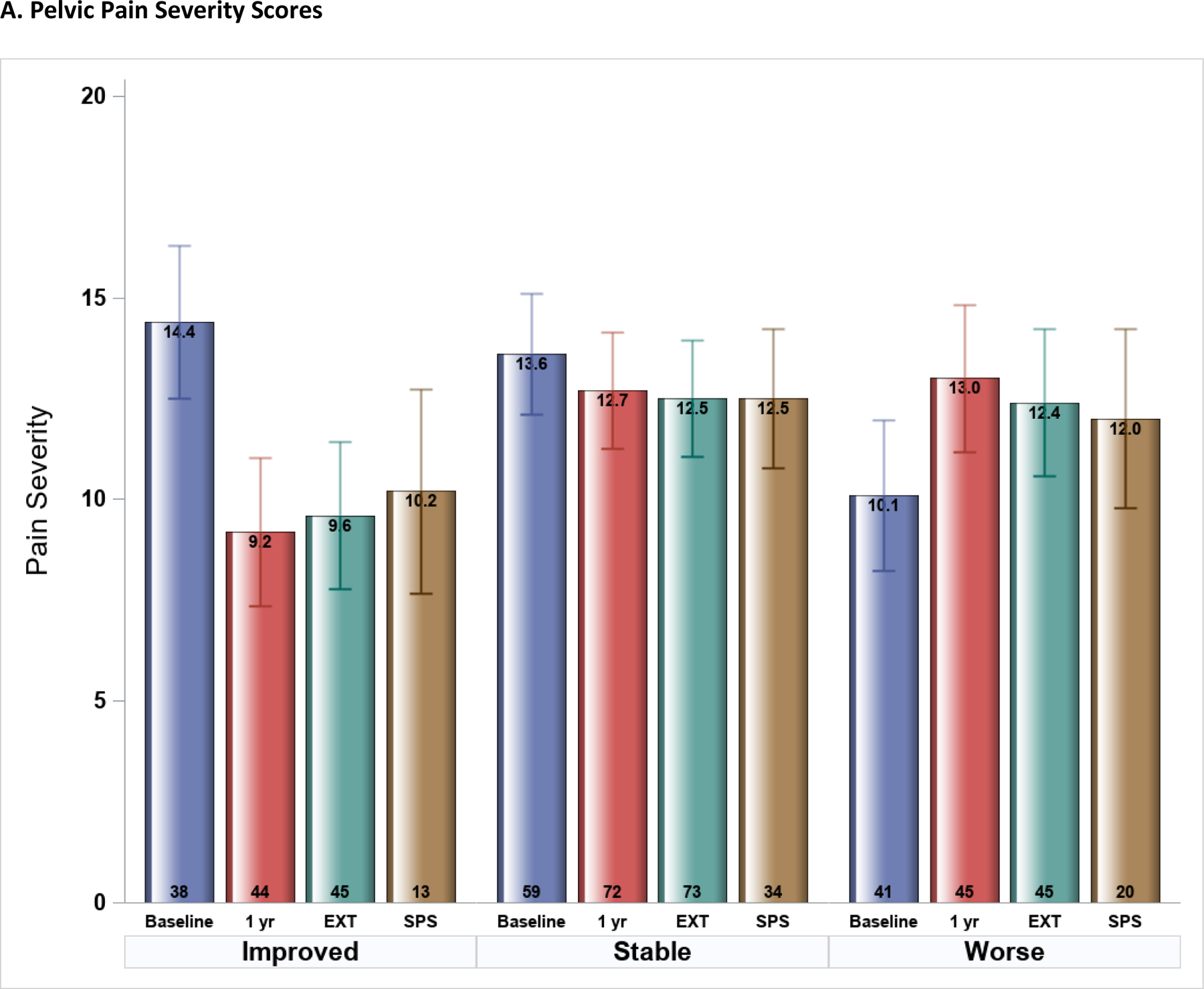
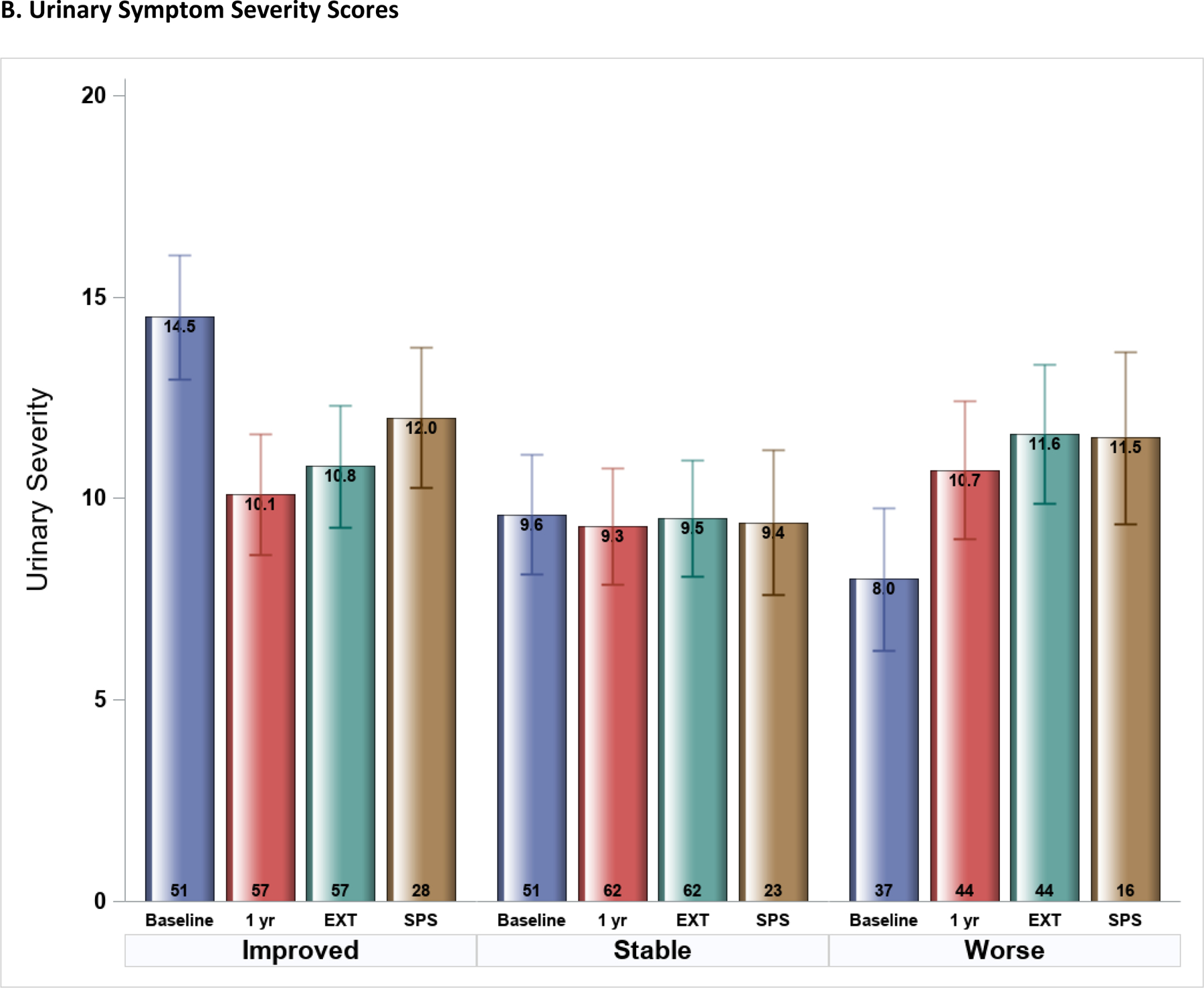
Symptom Severity scores at four study time-points (Epidemiology and Phenotyping Study Baseline and 1-year visits, Extension (EXT) phase, and Symptom Pattern Study (SPS) phase) stratified by 1-year symptom profiles (improved, stable, worse) (n=163). Mean (SE) scores shown were estimated from multi-phase mixed effects models, adjusted for baseline symptom severity. A. Pelvic Pain Severity scores, B. Urinary Symptom Severity scores.
Similar patterns were seen in USS during follow-up, but with less change after the initial 1-year study (Figure 2B). The 1-year improved group remained on average better during EXT and SPS (average score reduced by 3.7, p<0.0001, and 2.4, p=0.004, respectively, compared to baseline). Average scores for the stable group had no change at any time-point or phase during follow-up. The group who worsened in USS during the first year continued to have worse symptoms during the remainder of follow-up compared to baseline (mean score change (increase) of 3.6 and 3.5, p<0.001 for both, in EXT and SPS phases, respectively). Results from all PPS and USS score comparisons are included in Supplemental Figure 1. Analyses were repeated in the smaller group of participants (n=67) with complete follow-up (including both EXT and SPS), with very similar results as in the full cohort (Supplemental Figure 2).
Many baseline variables were associated with average scores per phase, but none were consistently associated with score change between the time-points and study phases. Detailed results from the models examining potential predictors of change in PPS and USS are presented in Supplemental Tables 1 and 2. Non-pelvic symptoms, catastrophizing, anxiety, depression, stress, and female sex were all associated with higher pain scores at almost all time-points/phases (p<0.01 for all; on-average associations across all time-points). Older age and better physical and mental health status were associated with lower pain scores (p<0.001 for all; on-average associations across all time-points). Non-pelvic symptoms, catastrophizing, and female sex were also associated with higher urinary scores and better physical health with lower urinary scores at most time-points/phases (p<0.05 for all; on-average associations across all time-points).
Discussion
UCPPS participants in this long-term observational study showed some symptom change during the first year after enrollment, but then minimal additional changes in either pain or urinary symptoms over the next 2–9 years. Those who improved in the first year maintained that level of improvement on average, and those who worsened remained worse on average during extended follow-up. About 40–45% of participants had stable pain and urinary average scores throughout follow-up. While clinical and psychosocial factors were associated with higher or lower pain and urinary scores across time-points/phases, none of the baseline characteristics examined were associated with additional worsening or improving symptoms past the first year of observation. For clinicians our results suggest there are stable categories of UCPPS patients, including those with mild, moderate, and severe symptoms and illness impact, that likely require different clinical management plans.
Sparse research data are available on long-term follow-up of UCPPS patients. The Interstitial Cystitis Database (ICDB) study followed over 600 patients with IC for a median of 31 months.2 Initial improvement (over the first 6 months) in pain, urgency and frequency outcomes was observed only in moderate to severe patients, and like our results, no further change in symptom outcomes was seen in up to 4 years’ follow-up. Similarly, a 2-year longitudinal study in 445 men with CP/CPPS found most symptom improvement occurred in the first 3 months, with little additional change at 2 years.3 Warren et al4 followed 304 women with IC/BPS for a median of 33 months. Compared to our results, slightly more women reported symptom improvement at last follow-up (35%), but the time course of improvement appeared to be more gradual. Different study populations may explain the different symptom patterns, as this study enrolled incident cases (enrolled on average 9 months after symptoms started), while MAPP and ICDB participants had much longer symptom durations (8–9 years on average).
In a clinic-based study, Yeh et al5 enrolled 198 women with IC/BPS diagnosed at least 5 years prior. After a mean follow-up of 16.6 years, 59% had symptom improvement compared to baseline, including 12% remission. The time course of improvement was not studied. These results, also in IC/BPS patients with longer-term disease, appear to differ from ours. Patients enrolled represented only about 40% of all IC/BPS patients in that clinic, so may or may not be representative of a larger clinic-based population.
It is difficult to interpret why change was seen in our cohort over year 1, but not later. Logically, EPS enrolled UCPPS participants at different points in their disease course, but we previously found length of disease was not a predictor of 1-year symptom change.7 Several aspects of entering EPS may have led to early symptom changes. The high frequency of assessment in the first year might facilitate regression to the mean of those with higher or lower levels of baseline symptoms, which persisted throughout follow-up. It is also possible EPS participation caused participants to focus more than usual on symptoms and behaviors, and this may have resulted in symptom change. Others have demonstrated how similar activities, such as completing a bladder diary, might act as treatment.22 Regardless of the reasons for the symptom changes seen in the first year, and despite intra-person week-to-week or month-to-month variability, the data clearly point to long-term stability in symptom severity over extended follow-up.
Strengths of our study include the prospective, multicenter design and adherence to standardized data collection methods, allowing us to analyze data collected for up to 9-year’s follow-up. To our knowledge, this represents the longest prospective study of IC/BPS and CP/CPPS symptom trajectories. Limitations of our work include our inability to adjust for treatment, as our studies were observational and detailed treatment information was not collected in EPS. The lack of longitudinal symptom change also limited our ability to study potential predictors of long-term symptom change. Finally, our results may not be generalizable to UCPPS patients seen in settings outside academic, tertiary care facilities, who, perhaps, may have more variable symptoms.
Conclusions
Women and men with UCPPS enrolled in MAPP Research Network studies showed a high degree of stability in both pain and urinary symptom severity over 2–9 years, following the initial 1-year of follow-up. This symptom stability was observed irrespective of their initial 1-year symptom trajectory and initial symptom severity. These results suggest UCPPS is a chronic condition with relatively stable pain and urinary symptoms over multiple years of follow-up.
Supplementary Material
Highlights.
Women and men with UCPPS showed remarkable stability in pain and urinary symptom severity for up to 9 years, irrespective of their initial symptom trajectory
Most of the change in the severity of pain and urinary symptoms occurred during the first year of follow-up.
About 40–45% of participants had stable pain and urinary symptom scores throughout follow-up.
Our results suggest there are stable categories of UCPPS patients, including those with mild, moderate, and severe symptoms and illness impact, that may require different clinical management plans.
Funding information:
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH)
Grant/Award Numbers: U01 DK082315, U01 DK082316, U01 DK082325, U01 DK082333, U01 K082342, U01 DK082344, U01 DK082345, U01 DK082370, U01 DK103227, U01 DK103260, U01 DK103271
Abbreviation Key
- CPPS
Chronic Prostatitis/Chronic Pelvic Pain Syndrome
- EPS
Epidemiology and Phenotyping Study
- EXT
EPS Extension Study
- GUPI
Genitourinary Pain Index
- IC/BPS
Interstitial Cystitis/Bladder Pain Syndrome
- ICDB
Interstitial Cystitis Database
- ICSI
Interstitial Cystitis Symptom Index
- IQR
interquartile range
- MAPP
Multidisciplinary Approach to the Study of Chronic Pelvic Pain
- PPS
Pelvic Pain Severity
- QOL
Quality of Life
- SPS
Symptom Patterns Study
- SD
Standard deviation
- UCPPS
Urologic Chronic Pelvic Pain Syndrome
- USS
Urinary Symptom Severity
Contributor Information
Catherine S. Bradley, Department of Obstetrics and Gynecology, Carver College of Medicine, University of Iowa, Iowa City, IA.
Robert Gallop, Department of Mathematics, Applied Statistics Program, West Chester University, West Chester, PA.
Siobhan Sutcliffe, Departments of Surgery (Public Health Sciences) and Obstetrics and Gynecology, Washington University School of Medicine, St. Louis, MO.
Karl J. Kreder, Department of Urology, Carver College of Medicine, University of Iowa, Iowa City, IA.
H. Henry Lai, Departments of Surgery (Urology) and Anesthesiology, Washington University School of Medicine, St Louis, MO.
J. Quentin Clemens, Department of Urology, University of Michigan, Ann Arbor, MI.
Bruce D. Naliboff, Department of Medicine, David Geffen School of Medicine at UCLA, Los Angeles, CA.
References
- 1.Hanno PM, Erickson D, Moldwin R et al. : Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol, 193: 1545–53, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Propert KJ, Schaeffer AJ, Brensinger CM et al. : A prospective study of interstitial cystitis: results of longitudinal followup of the interstitial cystitis data base cohort. The Interstitial Cystitis Data Base Study Group. J Urol, 163: 1434–9, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Propert KJ, McNaughton-Collins M, Leiby BE et al. : A prospective study of symptoms and quality of life in men with chronic prostatitis/chronic pelvic pain syndrome: the National Institutes of Health Chronic Prostatitis Cohort study. J Urol, 175: 619–23, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Warren JW, Greenberg P, Diggs C et al. : A prospective early history of incident interstitial cystitis/painful bladder syndrome. J Urol, 184: 2333–8, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Yeh HL, Jhang JF, Kuo YC et al. : Long-term outcome and symptom improvement in patients with interstitial cystitis/bladder pain syndrome with or without regular follow-up and treatment. Neurourol Urodyn, 38: 1985–93, 2019. [DOI] [PubMed] [Google Scholar]
- 6.Landis JR, Williams DA, Lucia MS et al. : The MAPP research network: design, patient characterization and operations. BMC Urol, 14: 58 (1–17), 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naliboff BD, Stephens AJ, Lai HH et al. : Clinical and Psychosocial Predictors of Urological Chronic Pelvic Pain Symptom Change in 1 Year: A Prospective Study from the MAPP Research Network. J Urol, 198: 848–57, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemens JQ, Kutch JJ, Mayer EA et al. : The Multidisciplinary Approach to The Study of Chronic Pelvic Pain (MAPP) Research Network*: Design and implementation of the Symptom Patterns Study (SPS). Neurourol Urodyn, 39: 1803–14, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemens JQ, Calhoun EA, Litwin MS et al. : Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology, 74: 983–7, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Leary MP, Sant GR, Fowler FJ Jr. et al. : The interstitial cystitis symptom index and problem index. Urology, 49 (Suppl 5A): 58–63, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Griffith JW, Stephens-Shields AJ, Hou X et al. : Pain and Urinary Symptoms Should Not be Combined into a Single Score: Psychometric Findings from the MAPP Research Network. J Urol, 195: 949–54, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephens-Shields AJ, Clemens JQ, Jemielita T et al. : Symptom Variability and Early Symptom Regression in the MAPP Study: A Prospective Study of Urological Chronic Pelvic Pain Syndrome. J Urol, 196: 1450–5, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleeland C: The Brief Pain Inventory: User Guide Houston, TX: MD Anderson Cancer Center, 2009. [Google Scholar]
- 14.Williams DA, Schilling S: Advances in the assessment of fibromyalgia. Rheum Dis Clin North Am, 35: 339–57, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ware J Jr., Kosinski M, Keller SD: A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care, 34: 220–33, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Zigmond AS, Snaith RP: The hospital anxiety and depression scale. Acta Psychiatr Scand, 67: 361–70, 1983. [DOI] [PubMed] [Google Scholar]
- 17.Cohen S, Kamarck T, Mermelstein R: A global measure of perceived stress. J Health Soc Behav, 24: 385, 1983. [PubMed] [Google Scholar]
- 18.Keefe FJ, Brown GK, Wallston KA et al. : Coping with rheumatoid arthritis pain: catastrophizing as a maladaptive strategy. Pain, 37: 51–6, 1989. [DOI] [PubMed] [Google Scholar]
- 19.Guo W, You M, Yi J et al. Functional Mixed Effects Clustering with Application to Longitudinal Urologic Chronic Pelvic Pain Syndrome Symptom Data, J Am Stat Assoc, Published ahead of print, DOI: 10.1080/01621459.2022.2066536 [DOI] [PMC free article] [PubMed]
- 20.Cudeck R, Klebe KJ: Multiphase mixed-effects models for repeated measures data. Psychol Methods, 7: 41–63, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Cudeck R, Harring JR: Analysis of nonlinear patterns of change with random coefficient models. Annu Rev Psychol, 58: 615–37, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Foster HE Jr., Hanno PM, Nickel JC et al. : Effect of amitriptyline on symptoms in treatment naïve patients with interstitial cystitis/painful bladder syndrome. J Urol, 183: 1853–8, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


