Abstract
Enterovirus D68 (EV-D68) can cause respiratory diseases and acute flaccid paralysis, posing a great threat to public health. Interferons are cytokines secreted by host cells that have broad-spectrum antiviral effects, inducing the expression of hundreds of interferon-stimulated genes (ISGs). EV-D68 activates ISG expression early in infection, but at a later stage, the virus suppresses ISG expression, a strategy evolved by EV-D68 to antagonize interferons. Here, we explore a host protein, suppressor of cytokine signaling 3 (SOCS3), is upregulated during EV-D68 infection and antagonizes the antiviral effects of type I interferon. We subsequently demonstrate that the structural protein of EV-D68 upregulated the expression of RFX7, a transcriptional regulator of SOCS3, leading to the upregulation of SOCS3 expression. Further exploration revealed that SOCS3 plays its role by inhibiting the phosphorylation of signal transducer and activator of transcription 3 (STAT3). The expression of SOCS3 inhibited the expression of ISG, thereby inhibiting the antiviral effect of type I interferon and promoting EV-D68 transcription, protein production, and viral titer. Notably, a truncated SOCS3, generated by deleting the kinase inhibitory region (KIR) domain, failed to promote replication and translation of EV-D68. Based on the above studies, we designed a short peptide named SOCS3 inhibitor, which can specifically bind and inhibit the KIR structural domain of SOCS3, significantly reducing the RNA and protein levels of EV-D68. In summary, our results demonstrated a novel mechanism by which EV-D68 inhibits ISG transcription and antagonizes the antiviral responses of host type I interferon.
Keywords: Enterovirus D68 (EV-D68), Interferon, ISG, SOCS3, STAT3
Highlights
-
•
The structural protein of EV-D68 up-regulates SOCS3 expression and inhibits STAT3 phosphorylation.
-
•
EV-D68 inhibit IFN-β-activated ISG by upregulating SOCS3 expression.
-
•
The structural protein of EV-D68 upregulates SOCS3 expression by upregulating RFX7 expression.
-
•
A short peptide called SOCS3 inhibitor that binds to the KIR domain of SOCS3 inhibits EV-D68 replication.
1. Introduction
Enterovirus D68 (EV-D68) belongs to the Picornaviridae family and is a single-stranded, positive-sense RNA virus (Hoorn and Tyrrell, 1965; Holm-Hansen et al., 2016). The EV-D68 genome encodes a precursor polyprotein that is further cleaved into four structural proteins (VP1 to VP4) and seven nonstructural proteins (2A, 2B, 2C, 3A, 3B, 3C, and 3D) (Smura et al., 2007; Opanda et al., 2014). EV-D68 predominantly causes respiratory disease (Hu et al., 2020). Optimal EV-D68 replication occurs at 33 °C (the temperature of the respiratory tract), which is similar to that of rhinoviruses and may be related to its respiratory tropism (Oberste et al., 2004). EV-D68 can also lead to severe clinical manifestations in the neurological system, such as acute flaccid myelitis and meningitis (Kohil et al., 2021; Ayudhya et al., 2022). A meta-analysis in 2022 showed that the case fatality rate due to EV-D68 infections ranged from 0 to 4.4% (Fall et al., 2022). Although EV-D68 was first isolated in 1962 (Holm-Hansen et al., 2016; Ebada et al., 2021), due to the mild symptoms of infection and the small number of people seeking medical treatment at that time, less research on its pathogenic mechanism has been conducted. However, since the EV-D68 outbreak in North America in 2014, the virus has gradually attracted the attention of researchers (Skowronski et al., 2015; Holm-Hansen et al., 2016; Hodcroft et al., 2022). Studies on how EV-D68 infects cells and evades host immunity provide major insights into novel preventive and therapeutic strategies.
Interferons (IFNs) are broad-spectrum antiviral molecules that can be divided into three categories: type I, II, and III (Negishi et al., 2018). Of these three, type I IFNs were discovered in the 1950s and comprise IFN-α, IFN-β, IFN-κ, IFN-ω, and IFN-ε. Type II IFNs only comprise IFN-γ. Additionally, type III IFNs comprise IFN-λ1 (IL-29), IFN-λ2 (IL-28a), and IFN-λ3 (IL-28b) (Capobianchi et al., 2015). Type II IFN has a strong immune regulation effect but weak antiviral ability. In contrast to type II interferon, both type I and type III interferons can activate the expression of interferon-stimulated genes (ISGs), which may be one of the reasons why they can inhibit EV-D68 replication. As the name suggests, ISG is induced by IFN and has multiple functions, especially in pathogen resistance and control. EV-D68 employs multiple strategies to block IFNs effects. For instance, EV-D68's 3Cpro can cleave the TIR domain-containing adaptor inducing beta interferon (TRIF) and block toll-like receptor 3 (TLR3)-mediated IFN synthesis (Xiang et al., 2014). Similarly, 3Cpro mediates the cleavage of IFN regulatory factor 7 (IRF7) and reduces IFN production (Xiang et al., 2016). Likewise, the viral protein 2Apro evaded the IFN system by cleaving tumor necrosis factor receptor-associated factor 3 (TRAF3) (Kang et al., 2021). Other viral proteins besides the above proteases can also affect IFN synthesis. For instance, 2C binds to p65 and inhibits the NF-κB pathway, and 3Dpol influences the retinoic acid-inducible gene 1 (RIG-I) pathway (Du et al., 2015; Lei et al., 2016; Yang et al., 2021). Notably, all these strategies employed by EV-D68 converge to impact IFN synthesis. Following viral detection, the host cells induces IFN synthesis, which is then transported out of the cell and binds to the cell surface receptors. It then transmits signals to the nucleus via the Janus kinases (JAK)-signal transducer and activator of transcription (STAT) signaling pathway. This raises the question of whether EV-D68 can attenuate the antiviral effects of IFN by blocking the JAK-STAT signaling pathway.
The JAK-STAT pathway consists of Janus kinases, signal transducer and activator of transcription family proteins, and tyrosine-protein kinases (Yokota et al., 2004). The JAK family consists of JAK1, JAK2, JAK3, and TYK2, whereas the STAT family consists of STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6 (Murray, 2007). IFNs bind to receptors on the cell surface (e.g., type I IFNs bind IFNAR), resulting in the phosphorylation of JAKs and subsequent phosphorylation of STATs. Phosphorylated STAT (p-STAT) then forms dimers that enters the nucleus, inducing IFN-stimulated gene (ISG) transcription with antiviral effects (Liongue et al., 2012; Harrison and Moseley, 2020). Our previous study showed that EV-D68 infection induced the accumulation of SG marker proteins Ras GTPase-activated protein-binding protein 1 (G3BP1), T cell intracellular antigen 1 (TIA1), and human antigen R (HUR) in the cytoplasm of infected host cells during early infection but inhibited their accumulation during the late stage (Cheng et al., 2020). This observation suggests the existence of a strategy by which EV-D68 antagonizes host ISG transcription by EV-D68.
The SOCS family represents a well-known feedback inhibitor of the JAK-STAT signaling pathway (Croker et al., 2008). The SOCS family consists of eight structurally similar proteins: suppressor of cytokine signaling 1–7 (SOCS1–7) and cytokine-inducible SH2-containing protein (CIS) (Gao et al., 2018). These proteins have the following structures: the N-terminal with varying amino acid lengths, the central region with the SH2 domain, and the C-terminal with the SOCS box. The SH2 structural domain of SOCS3 can bind to cell surface receptor phosphotyrosine residues and block cell surface receptor-induced JAK phosphorylation, and it also can bind to phosphotyrosine residues on the JAK surface and inhibit downstream signaling (Linossi et al., 2018; Khan et al., 2019). SOCS BOX can bind to Elongin B, Elongin C, and Cullin5 proteins to form E3 complexes and activate ubiquitinated degradation pathways to degrade JAK proteins bound to SOCS (Hsia et al., 2017). Furthermore, SOCS1 and SOCS3 also have another kinase inhibitory region (KIR) domain that can inhibit JAK tyrosine kinase activity by acting as a pseudo-substrate (Wei et al., 2019; Xie et al., 2021).
This study aimed to investigate the mechanism by which EV-D68 utilize SOCS3 to antagonize the type I interferon pathway. Our findings reveal that viral-induced SOCS3 upregulation inhibits STAT3 phosphorylation, and antagonizes the antiviral effects of IFNβ, thereby promoting transcription, protein, and viral titer levels of EV-D68. We further detail the molecular mechanisms of viral-induced SOCS3 upregulation. Finally, we designed a short peptide named SOCS3 inhibitor, which specifically binds and inhibits the KIR structural domain of SOCS3. The findings from this study increase our understanding of the mechanism of EV-D68 antagonize type I interferon and provide a new intervention strategy for combating viral infection.
2. Materials and methods
2.1. Cells, viruses, and plasmids
RD human malignant embryonic rhabdomyoma cells (RD cells), HEK293T human embryonic kidney 293 cells (293T cells), and HeLa cervical carcinoma cells (HeLa cells) were obtained from the American Type Culture Collection (ATCC). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; 11995500BT, Thermo Fisher, USA) supplemented with 10% fetal bovine serum (FBS; FSP500-12B031, Excell, China) and 1% penicillin-streptomycin solution (SV30010, Cytiva, USA) at 37 °C in a 5% CO2 incubator.
The EV-D68 prototype Fermon strain (NCBI Reference Sequence: NC_038308.1) was obtained from Xiaofang Yu (Cancer Center, Zhejiang University)(Wei et al., 2016; Schieble et al., 1967). The EV-A71 Fuyang strain (GenBank accession no. FJ439769.1) was obtained from Fei Guo (MOH Key Laboratory of Systems Biology of Pathogens, Institute of Pathogen Biology, and Center for AIDS Research, Chinese Academy of Medical Sciences &Peking Union Medical College) (Xu et al., 2016).
The pCAGGS vector was deposited in our laboratory. The pCAGGS-SOCS-flag plasmid and its corresponding truncated plasmids (ΔKIR, ΔSH2-Q, ΔSH2-H, and ΔBOX) were constructed by the authors.
2.2. Antibodies and reagents
SOCS3 Rabbit pAb (A0694) and SOCS3 Rabbit pAb (38150) were purchased from ABclonal (Wuhan, China) and SAB (Nanjing, China), respectively. The STAT3 Rabbit pAb (A1192), Phospho-STAT3-Y705 Rabbit mAb (AP0705), and Phospho-STAT1-S727 Rabbit mAb (AP1000) were purchased from ABclonal (Wuhan, China). Phospho-STAT1 (Tyr7010) Rabbit pAb was purchased from Affinity (Nanjing, China). DYKDDDDK tag Rabbit Polyclonal Antibody (20543-1-AP) and HA tag Rabbit Polyclonal Antibody (51064-1-AP) were purchased from Proteintech (Wuhan, China). β-Tubulin Mouse mAb (M9003) was purchased from Simu Biotechnology (Tianjin, China). Enterovirus D68 VP1 Rabbit pAb was purchased from GeneTex (Beijing, China). An Enterovirus D68 VP1 Mouse mAb was manufactured at our laboratory.
The recombinant IFN proteins used in this study were purchased from Sino Biological Inc, Beijing, with the following product numbers: recombinant IFN-β (10704-HNAS, SinoBiological, China).
2.3. RNA extraction and qRT-PCR
Total RNA was extracted using TRIzol reagent (391,312, Ambion, USA) and reverse-transcribed into cDNA using the Hifair® III 1st Strand cDNA Synthesis Kit (11139ES10; Yeasen, China). Subsequently, cDNA was subjected to qPCR to detect changes in mRNA levels using Hieff® qPCR SYBR Green Master Mix (11201ES03; Yeasen, China). The results were repeated three times and analyzed as fold changes using the 2−ΔΔCt method.
The qPCR primers for genes are shown in Supplementary Table S1.
2.4. Western blot
Total protein was extracted from whole cells using RIPA lysis buffer containing protease inhibitors (4693132001-20, Roche, Switzerland). Equal amounts of protein were separated on SDS-PAGE and transferred to NT membranes (6590786, PALL Corporation, USA). After blocking with 5% skim milk for 1 h at 25 °C, the membranes were incubated with primary antibodies at 25 °C for 2 h, followed by an HRP-linked secondary antibody at 25 °C for 1 h. The samples were detected using an enhanced chemiluminescence detection system (36208ES60, Yeasen, China), and the intensity of protein expression was quantified using ImageJ software.
2.5. siRNA knockdown
siRNA targeting SOCS3 with the sequence 5´-GACUCCUACGAGAAGAUGATT-3´ was purchased from Hanbio (Shanghai, China). siRNA was transfected into cells using Lipo 2000 (2191415, Invitrogen, USA) according to the manufacturer's instructions.
2.6. Immunofluorescence experiments
The immunofluorescence experiments were carried out as follows. The cultuture medium was discarded, and cells were washed twice with PBS. Subsequently, cells were fixed with 4% paraformaldehyde for 15 min, followed by two washes with PBS. After fixation, cells were permeabilized with 0.5% Triton X-100 for 10 min and then washed twice with PBS. A blocking step was performed using 1% BSA Block at 25 °C for 20 min. The blocking solution was then removed and primary antibody dilution (1:200) was added to each well and incubated at 4 °C for 8 h. Subsequently, the primary antibody dilution was discarded and wells were washed five times for 1 min each with TBST. Next, a fluorescent secondary antibody dilution solution (1:400) was added to each well and incubated at 37 °C for 40 min; and washed with TBST five times. Next, 10 μL DAPI was added to stain the nuclei in the dark. After three washes with double-distilled water (1 min each), 1.8 μL of 50% glycerol was dropped on the slide. The slide was gently lifted with tweezers and inverted onto a cover slide for observation using an upright fluorescence microscope.
2.7. Data analysis
The data are presented as the mean ± standard deviation (SD) from three independent experiments. Statistical comparisons between treatment groups were carried out using the unpaired, two-tailed Student's t-test. P values were calculated using GraphPad's QuickCalc software (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001).
3. Results
3.1. EV-D68 inhibits the JAK-STAT signaling pathway
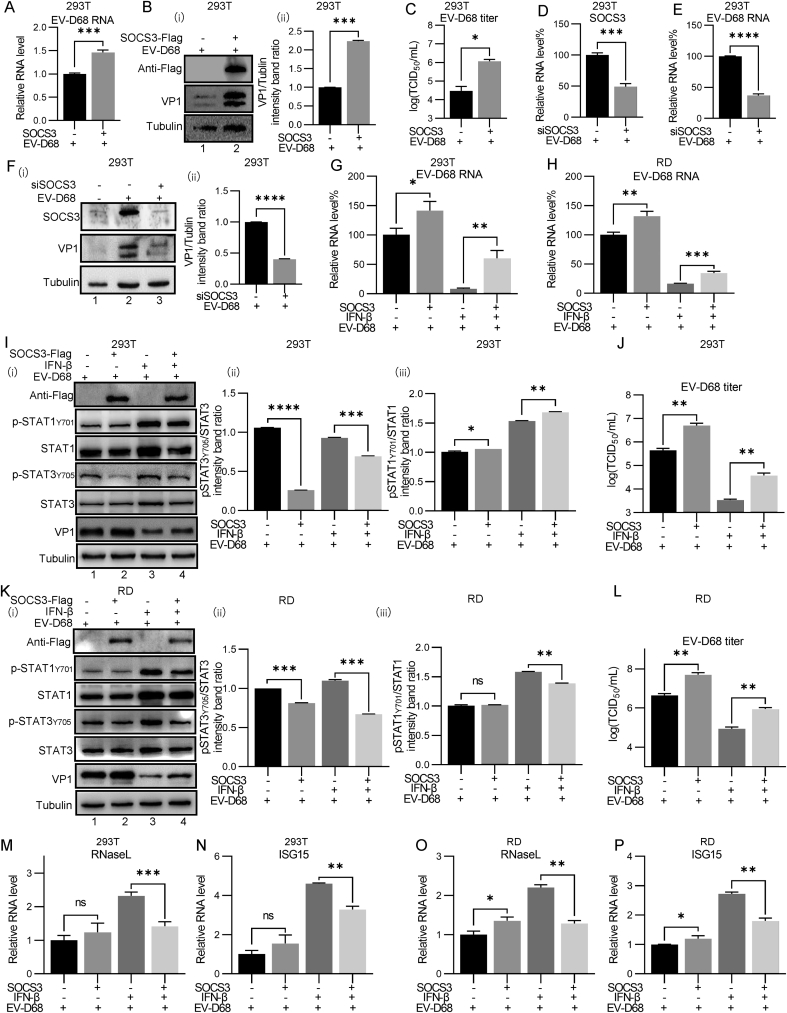
Interferons are broad-spectrum antiviral molecules. Type I interferons can activate the expression of interferon-stimulated genes (ISGs), which may be one of the reasons why they can inhibit EV-D68 replication. IFN-β can significantly inhibit EV-D68 replication at both protein and mRNA levels (Fig. 1A–D). It also significantly upregulates STAT1 and STAT3 expression and phosphorylation at Y701, S727, and Y705 amino acid residues (Fig. 1E–G). Moreover, IFN-β activates ISGs such as RNase L and ISG15 (the first and third bars in Fig. 1H–K). In contrast, EV-D68 infection only slightly activates ISG expression (the first and second bars in Fig. 1H–K). Notably, in the presence of sufficient amounts of IFN, infection with EV-D68 also inhibited ISG synthesis (the third and fourth bars in Fig. 1H–K). These findings suggest that EV-D68 effectively counters the antiviral effects of IFN-β. Therefore, our subsequent studies will focus on demonstrating EV-D68's mechanisms for antagonizing IFN-β.
Fig. 1.
EV-D68 inhibits the JAK-STAT signaling pathway. A–D HEK293T and RD cells were pretreated with IFN-β for 6 h, and the cells were infected with EV-D68 for 12 h, followed by cell collection. RNA levels of EV-D68 were detected by qRT-PCR, and protein levels of EV-D68 were detected by Western blot. E–G Cells were collected after treating RD cells with IFNβ for 12 h. The protein levels of STAT1, STAT3, p-STAT1 Y701, p-STAT1 S727, p-STAT3 Y705 were detected by Western blot. H–I Pretreatment of HEK293T and RD cells with IFN-β for 6 h, infection of cells with EV-D68 for 12 h, followed by cell collection. The mRNA levels of RNase L and ISG15 were detected by qRT-PCR. J Phosphorylationomics study of EV-D68 infection (partial data related to the JAK-STAT signaling pathway). The red and green bars indicate the ratio of phosphorylation levels at 12 and 24 h after EV-D68 infection to the levels in the non-infected group, respectively. K–L Differential expression of the JAK-STAT signaling pathway in transcriptomics and proteomics at 12 and 24 h of EV-D68 infection, respectively. The redder the color, the higher the expression of this gene after EV-D68 infection. Similarly, the bluer the color, the lower the expression of this gene. The results are presented as the mean and standard deviation of three independent experiments. Statistical significance was analyzed using the Student's t-test (∗ P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001, ns, not significant).
After IFN release from cells, it binds to interferon receptors on the cell membrane, triggering ISGs transcription via the JAK-STAT signaling pathway. Therefore, EV-D68 is suspected of disrupting the JAK-STAT signaling pathway and suppressing the activation of ISGs. Therefore, transcriptomic, proteomic, and phosphorylomic analyses of EV-D68 infection were performed in this study. Subsequently, data related to the JAK-STAT signaling pathway in the three omics datasets were extracted for further analysis. Phosphorylationomics data showed that phosphorylation levels at the STAT3 Y705 site in the EV-D68-infected group were 65% (12 h) or 35% (24 h) of those in the mock group (Fig. 1L). In addition, Fig. 1F and G shows the transcriptomic and proteomic data at different time points after EV-D68 infection. However, neither the RNA nor the protein levels of STAT3 changed significantly at 12 or 24 h after infection. Meanwhile, STAT1 and STAT2 showed no significant changes in the RNA, protein, and phosphorylation levels (Fig. 1L–N). It can be hypothesized that EV-D68 infection interferes with the normal function of STAT3 by affecting its phosphorylation levels (Fig. 1L–N). Notably, the expression of SOCS3, a negative regulator of the JAK-STAT signaling pathway, was upregulated in the transcriptomic data of EV-D68 infection (Fig. 1M). This suggests that EV-D68 may inhibit JAK-STAT signaling by upregulating SOCS3 expression.
3.2. EV-D68 infection upregulates SOCS3 expression and inhibits STAT3 phosphorylation
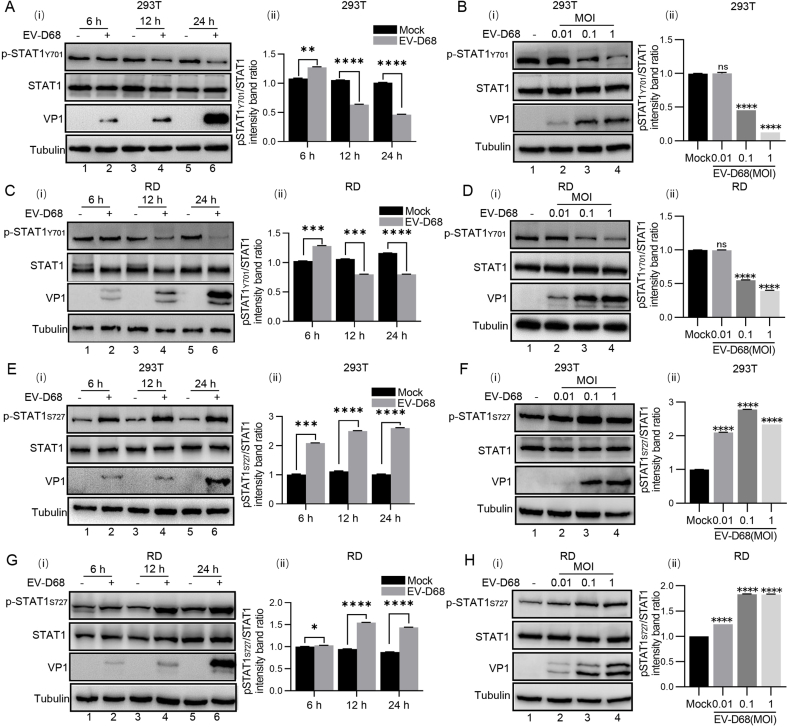
To clarify the interaction between EV-D68 and the JAK-STAT3 pathway, HEK293T cells and RD cells were infected with EV-D68 (MOI = 0.1) and collected at different times (6, 12, and 24 h) for analysis. Western blot results showed that the phosphorylation level of STAT3 significantly decreased with increasing infection duration, while the level of STAT3 remained unchanged (Fig. 2A and C). A similar reduction effect was observed in EV-D68-infected HEK293T and RD cells at different MOIs for 24 h (MOI = 0.01, 0.1, 1) (Fig. 2B and D). Briefly, EV-D68 infection did not affect the protein content of STAT3 but decreased its phosphorylation level.
Fig. 2.
EV-D68 upregulates the expression of SOCS3 and downregulates the phosphorylation level of STAT3. A–D HEK293T cells and RD cells were infected with EV-D68 (MOI = 0.1) at various times (6, 12, 24 h), and then the cells were collected. EV-D68-infected HEK293T cells and RD cells at different MOI (0.01, 0.1, and 1) for 24 h, and then cells were collected. The protein levels of STAT3 and p-STAT3 Y705 were measured by Western blot. E–L HEK293T cells and RD cells were infected with EV-D68 (MOI = 0.1) at various times (6, 12, 24 h), and then the cells were collected. EV-D68-infected HEK293T cells and RD cells at different MOI (0.01, 0.1, and 1) for 24 h, and then cells were collected. RNA levels of SOCS3 were detected by qRT-PCR, and protein levels of SOCS3 were detected by Western blot. Grayscale analysis of the Western blot results was performed using ImageJ software; three independent grayscale analyses were performed, and the results were averaged to ensure their reliability. qRT-PCR results are presented as the mean and standard deviation from three independent experiments. Statistical significance was analyzed using the Student's t-test (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001, ns, not significant).
We also examined the effects of EV-D68 infection on STAT1 protein and phosphorylation levels. EV-D68 infection had no significant effect on the protein content of STAT1 but inhibited phosphorylation at the STAT1 Y701 site and promoted phosphorylation at the STAT1 S727 site with increasing time (6, 12, and 24 h) and a gradient of viral MOI (MOI = 0.01, 0.1, 1) (Supplementary Figs. S1A and S1H).
Meanwhile, qRT-PCR results showed that mRNA levels of SOCS3 were upregulated in a time- and dose-dependent manner after virus infection (Fig. 2E–H). Western blot also showed the same results. The protein content of SOCS3 was elevated with the increase of infection time and dose (Fig. 2I–L). The above findings confirmed that EV-D68 upregulates SOCS3 expression to inhibit STAT3 phosphorylation.
3.3. SOCS3 inhibits type I interferon signaling to positively regulate the replication level of EV-D68
As a negative regulator of the JAK-STAT3 signaling pathway, SOCS3 can induce a decrease in the phosphorylation level of STAT3. Given that interferon signaling depends on the JAK-STAT pathway, the upregulated expression of SOCS3 by EV-D68 may hinder the antiviral effect of interferon.
The results of qRT-PCR, Western blot, and TCID50 analysis showed that overexpression of SOCS3 in 293T cells was beneficial to the replication of EV-D68, promoting the replication of EV-D68 at RNA and viral titer levels by approximately 1.5 times and protein levels by approximately 2.25 times (Fig. 3A–C).
To further assess this, we knocked down the intracellular SOCS3 levels using siRNA, and examined its effect on EV-D68 replication. The experimental results showed successful SOCS3 knockdown by siRNA, and the RNA level of SOCS3 was only 50% of the control group (Fig. 3D). At the same time, the reduction in SOCS3 also reduced the RNA level of EV-D68 to approximately 40% of the control group (Fig. 3E). Western blot experiments also reached similar conclusions: EV-D68 infection upregulated the expression of SOCS3 compared with the mock group (Fig. 3F, lanes 1 and 2). Reducing the level of SOCS3 with siRNA reduced the protein level of EV-D68 by approximately 50% (Fig. 3F, lanes 2 and 3). These results suggest that reduced SOCS3 expression is detrimental to EV-D68 replication.
Fig. 3.
SOCS3 inhibits type I interferon-induced ISG expression to positively regulate the replication level of EV-D68. A–C HEK293T cells were transfected with the pCAGGS empty vector and the pCAGGS-SOCS3-flag plasmid for 24 h and then infected with EV-D68 (MOI = 0.1) for 24 h. The cells were collected for qRT-PCR, Western blot, and detection of the TCID50 virus titer. D–F HEK293T cells were transfected into 10 μmol/L siRNA (siSOCS3) for 48 h; cells were then infected with EV-D68 (MOI = 0.1) and incubated for 12 h. Cells were collected and subjected to qRT-PCR (D, E) and Western blot (F) to detect the RNA levels of SOCS3 and EV-D68 and protein levels of EV-D68. G–P SOCS3 was transfected into HEK293T and RD cells, and 24 h later, the cells were treated with IFN-β for 6 h. Subsequently, the cells were infected with EV-D68 (MOI = 0.1) and incubated for 12 h. Cells were collected and subjected to qRT-PCR (G, H), Western blot (I, K), or viral titers (J, L) to detect EV-D68 RNA, protein, and titer levels. In addition, cells were collected and simultaneously subjected to qRT-PCR assays for ISG gene (RNaseL and ISG15) expression (M–P). Grayscale analysis of the Western blot results was performed using ImageJ software; three independent grayscale analyses were performed, and the results were averaged to ensure their reliability. qRT-PCR results are presented as the mean and standard deviation from three independent experiments. Statistical significance was analyzed using the Student's t-test (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001, ns, not significant).
Further, the mechanism by which SOCS3 helps EV-D68 replication was explored. The addition of IFN-β resulted in a significant decrease in EV-D68 replication levels. Notably, overexpression of SOCS3 significantly attenuated the antiviral effect of IFN-β, increasing viral replication levels by approximately 7-fold in HEK293T cells and about 2-fold in RD cells under IFN-β stress (Fig. 3G and H). Consistently, using Western blot and TCID50 assays, similar conclusions were obtained at the protein level (Fig. 3I and K) and virus titer levels (Fig. 3J and L), respectively. Notably, overexpression of SOCS3 downregulated phosphorylation at the STAT3 Y705 site but not at the STAT1 Y701 site (Fig. 3I and K, lanes 1 and 2), further demonstrating that upregulation of SOCS3 expression by EV-D68 promotes viral replication by inhibiting the phosphorylation of STAT3 but not STAT1. However, in the presence of IFNβ, overexpression of SOCS3 downregulated both STAT1 and STAT3 protein and phosphorylation levels. This may be due to the higher replication and translation level of EV-D68 in the SOCS3 overexpression experimental group, resulting in stronger inhibition of EV-D68 on IFNβ and weakening the up-regulatory effect of IFNβ on STAT1 and STAT3 in this experimental group (Fig. 3I and K, lanes 3 and 4).
In addition, IFN-β activated the JAK-STAT signaling pathway and promoted the expression of ISG, such as RNase L and ISG15. However, SOCS3 overexpression reduced ISG activation by IFN-β. It is worth noting that without the addition of IFN-β, the expression of ISG in the SOCS3 overexpression group was higher than that in the control group, which could be attributed to heightenedviral replication caused by SOCS3, leading to increased interferon levels and consequent ISG expression (Fig. 3M−P).
3.4. Structural protein of EV-D68 upregulates SOCS3 expression by upregulating RFX7 expression
As mentioned above, EV-D68 infection can upregulate SOCS3 expression in host cells at both the RNA and protein levels. However, the mechanism by which EV-D68 upregulates this gene remains unclear and requires further investigation.
Huang et al. (2021) showed that miR-103/miR-107 inhibited the replication of Enterovirus A71 (EV-A71) and promoted the type I interferon response by regulating the SOCS3-STAT3 pathway. miR-103 and miR-107 were found to directly target SOCS3. The downregulation of these two miRNAs leads to the upregulation of SOCS3 expression and further affects STAT3 phosphorylation (Huang et al., 2021). EV-A71 and EV-D68 are both enteroviruses; therefore, does EV-D68 affect SOCS3 expression through the same mechanism?
In our study, HEK293T cells were infected with EV-D68 and EV-A71 and collected at the indicated time points for RT-qPCR analysis. The results showed that the replication levels of both EV-A71 (Fig. 4A) and EV-D68 (Fig. 4D) increased with increasing infection time. The expression levels of miR-103 and miR-107 were downregulated in the host cells infected with EV-A71 (Fig. 4B and C). However, in contrast to the results of EV-A71 infection, miR-103 and miR-107 expression levels in host cells were downregulated only at the early stage of EV-D68 infection, namely, at 6 h, and were upregulated at 12 and 24 h (Fig. 4E and F). The changes in miR-103 and miR-107 levels after cell infection with EV-D68 showed different trends from those after infection with EV-A71, suggesting that EV-D68 infection upregulates SOCS3 levels differently from that of EV-A71. Expression plasmids containing EV-D68's four structural proteins (VP1, VP2, VP3, and VP4) and seven non-structural proteins (2A, 2B, 2C, 3A, 3B, 3C, and 3D) were constructed. They were transfected into HEK293T cells, and SOCS3 and STAT3 phosphorylation levels in the cells were detected. It was shown that nonstructural proteins failed to significantly upregulate SOCS3 expression and that 3C and 3D proteins downregulated SOCS3 expression (Fig. 4G). In contrast, expression of the three structural proteins, VP1, VP2/4, and VP3, resulted in the upregulation of SOCS3 levels at both the RNA and protein levels (Fig. 4H and I). These structural proteins also inhibited the phosphorylation of STAT3 (Fig. 4I). Moreover, UV-inactivated EV-D68 can also upregulate SOCS3 at the RNA level (Fig. 4J). Further studies showed that the transcription of two ISG genes, RNase L and ISG15, which are activated by IFNβ, was inhibited by the structural protein of EV-D68 (Fig. 4K and L).
Fig. 4.
Structural protein of EV-D68 upregulates SOCS3 expression by upregulating RFX7 expression A-C HEK293T cells were infected with EV-A71 viruses, and cells were collected after 6, 12, and 24 h of viral infection; RNA was extracted and reverse-transcribed using the stem-loop method. The viral mRNA levels of EV-A71 (A), miR-103 (B), and miR-107 (C) in host cells were detected by qRT-PCR. D–F HEK293T cells were infected with EV-D68 viruses, collected at 6, 12, and 24 h of viral infection, and RNA was extracted and reverse-transcribed using the stem-loop method. The viral mRNA levels of EV-D68 (D), miR-103 (E), and miR-107 (F) in host cells were detected by qRT-PCR. G HEK293T cells were transfected with expression plasmids of EV-D68 non-structural proteins 2A, 2B, 2C, 3A, 3AB, 3C, and 3D. After 48 h of culture, cells were collected and analyzed for SOCS3 protein levels. Red box indicated the targeted protein band. H–I HEK293T cells were transfected with expression plasmids for EV-D68 structural proteins VP1, VP2/4 (also called VP0, cleaved into VP2 and VP4 during maturation), and VP3. After 48 h of culture, the cells were collected and analyzed for SOCS3 mRNA, protein, and STAT3 phosphorylation levels. J EV-D68 was inactivated by irradiation with UV light for 6 h. The UV-inactivated EV-D68 was infected with 293T cells for 24 h. The cells were collected and the RNA level of SOCS3 was detected by qRT-PCR. K–L Transfection of EV-D68 structural proteins VP1, VP2/4, and VP3 in RD cells for 24 h, then the cells were treated with IFN-β for 6 h, and the cells were collected for qRT-PCR to detect mRNA levels of ISG (RNaseL and ISG15). M RD cells were infected with EV-D68 (MOI = 0.1) at various times (6, 12, and 24 h), and the cells were collected for qRT-PCR to detect mRNA levels of RFX7. N Transfection of EV-D68 structural proteins VP1, VP2/4, and VP3 in RD cells, 48 h after transfection, the cells were collected for qRT-PCR to detect mRNA levels of RFX7. O-Q Transfection of RD cells with 50 μmol/L siRNA (siRFX7) for 48 h, cells were infected with EV-D68 (MOI = 0.1) and incubated for 12 h. Cells were collected and subjected to qRT-PCR (O, P) and Western blot (Q) to detect RNA levels and protein levels of SOCS3 and EV-D68. Grayscale analysis of the Western blot results was performed using ImageJ software; three independent grayscale analyses were performed, and the results were averaged to ensure their reliability. qRT-PCR results are presented as the mean and standard deviation from three independent experiments. Statistical significance was analyzed using the Student's t-test (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001, ns, not significant).
RFX7 is a transcription factor for SOCS3 (Wang et al., 2023); to investigate the underlying mechanism of EV-D68 upregulation of SOCS3 expression, we further investigated the effect of EV-D68 infection and structural proteins of EV-D68 on RFX7. The results of this study showed that both EV-D68 infection and overexpression of EV-D68 structural proteins upregulated the transcription levels of RFX7 (Fig. 4M and N). RFX7 knockdown downregulated the mRNA and protein levels of SOCS3 by approximately 50% (Fig. 4O–Q). Knockdown of RFX7 also suppressed protein levels of EV-D68 by approximately 40% (Fig. 4Q).
3.5. SOCS3 KIR regions are critical sites for promoting viral replication
SOCS3 has several crucial regions: the KIR domain (1–33), the SH2 domain (46–142), and the SOCS BOX region (177–225). Therefore, four SOCS3 truncating mutants, ΔKIR, ΔSH2-Q, ΔSH2-H, and ΔBOX, were constructed for subsequent studies (Fig. 5A). Next, HEK293T cells and RD cells were transfected with intact SOCS3 and these four SOCS3 truncating mutants for 24 h, and then infected with EV-D68 for 12 h. The results showed that SOCS3 and its ΔSH2-Q, ΔSH2-H, and ΔBOX truncating mutants all promoted the replication and translation of EV-D68, but the ΔKIR truncation mutant lost these effects (Fig. 5B–E).
Fig. 5.
SOCS3 inhibitor inhibited EV-D68's RNA and protein expression. A Schematic representation of SOCS3 truncating mutants. B–E Wildtype SOCS3, SOCS3 ΔKIR, SOCS3 ΔSH2-Q, SOCS3 ΔSH2-H, and SOCS3 ΔBOX were expressed in HEK293T cells and RD cells for 24 h, then infection with EV-D68 for 24 h, and the cells were collected. The RNA and protein levels of EV-D68 were detected by qRT-PCR (B,C) and Western blot (D,E). D Peptides, inhibitor-1 and inhibitor-2, were designed to specifically bind to the KIR domain of SOCS3 to inhibit its function. G Transfection of inhibitor-NC, inhibitor-1, and inhibitor-2 in HEK293T cells for 24 h. Cells were collected for the CCK8 assay to detect cell viability. H HeLa cells were transfected with the pCAGGS-SOCS3-flag plasmid, inhibitor-2 plasmid, or co-transfected with the above two plasmids. After 24 h, the cells were fixed, and the intracellular localization of SOCS3 and inhibitor-2 was detected by immunofluorescence. The line-scanning plots were used to quantify florescence intensity. I-L Transfection of inhibitor-NC, inhibitor-1, and inhibitor-2 in HEK293T cells and RD cells for 24 h, then the cells were treated with IFN-β for 6 h. Subsequently, the cells were infected with EV-D68 (MOI = 0.1) and incubated for 12 h. Cells were collected and subjected to qRT-PCR (I, J) and Western blot (K, L) to detect EV-D68 RNA protein levels. Grayscale analysis of the Western blot results was performed using ImageJ software; three independent grayscale analyses were performed, and the results were averaged to ensure their reliability. qRT-PCR results are presented as the mean and standard deviation from three independent experiments. Statistical significance was analyzed using the Student's t-test (∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001, ns, not significant).
The unique KIR domains of SOCS3 can specifically bind to the activation loop of JAK, and the amino acid sequence and protein structure of the latter have been reported. We used these properties to design a peptide (SOCS3 inhibitor-1; sequence LPQDKEYYKV-KEP) (Waiboci et al., 2007). This peptide competitively binds to the SOCS3 KIR domain, thereby inhibiting the normal function of SOCS3 (Fig. 5F). To enhance the inhibitor's effectiveness, a repeat of LPQDKEYYKVKEP was added to the C-terminus of SOCS3 inhibitor-1, and the two repeat peptides were linked by a P2A linker peptide, an HA tag was added to the N-terminus, and the newly constructed peptide is called SOCS3 inhibitor-2 (Fig. 5F). The P2A-conjugated peptide undergoes self-shearing and can be translated under the control of the same promoter to obtain two segments of protein with almost 100% cleavage efficiency. In addition, a negative control, SOCS3 inhibitor-NC, was devised through computer-randomized scrambling of the peptide's amino acid sequence to form the PKEEKYDYLQV sequence (Fig. 5F).
To investigate the effect of the SOCS3 inhibitor on cell viability, HEK293T cells were transfected with SOCS3, SOCS3 inhibitor-1, and SOCS3 inhibitor-2 for 24 h, followed by CCK8 assays. The results showed that the SOCS3 inhibitor did not reduce cell viability (Fig. 5G). To confirm the SOCS3 inhibitor's binding ability, HeLa cells were transfected with both SOCS3 and SOCS3 inhibitor-2 simultaneously or separately, and immunofluorescence analysis was performed to reveal colocalization of SOCS3 inhibitor-2 and SOCS3 (Fig. 5H). In addition, the effects of these peptides on EV-D68's replication and translation were verified. The results showed that both SOCS3 inhibitor-1 and SOCS3 inhibitor-2 inhibited the RNA and protein levels of EV-D68 in the absence and presence of IFN-β (Fig. 5I–L). Inhibitor-1 and inhibitor-2 were shown to bind to the KIR structural domain of SOCS3, thus contributing to their antiviral effect. This is due to antagonizing the ability of SOCS3 to inhibit STAT3 phosphorylation, ensuring proper conduction of the JAK-STAT3 pathway.
4. Discussion
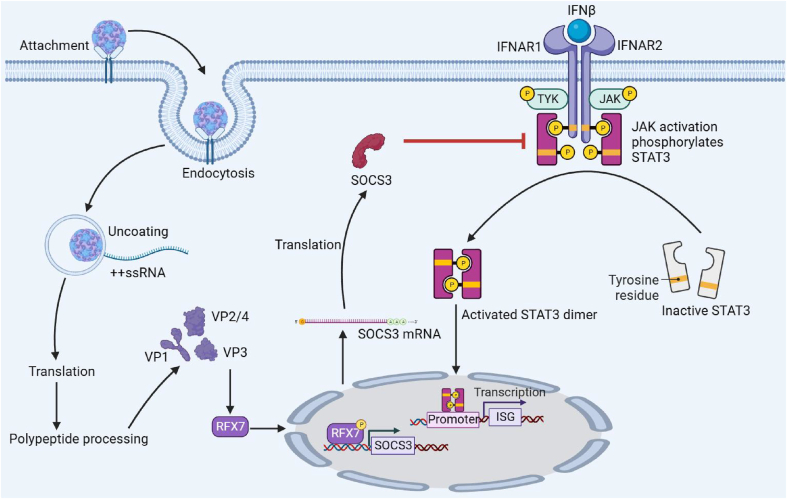
The host has evolved an interferon system to resist viral invasion. Intracellular pattern recognition receptors, such as TLR3, RIG-I, and MDA-5, detect dsRNA, 5´ppp RNA, and long RNA produced during EV-D68 replication and activate the adaptor molecules TRIF or MAVS. The signal is further transmitted to kinases such as TBK1 and IKK complexes, which activate transcription factors such as IRF3, IRF7, and NF-κB and initiate the transcription of IFN-β. Subsequently, the translated IFN-β molecules are released outside the cell membrane, and after being recognized by the interferon recognition receptors on the cell surface, the phosphorylation of JAK and STAT3 is activated. Phosphorylated STAT3 forms a dimer and enters the nucleus. However, EV-D68 has also developed various strategies to evade the host's immune response. As described in the Introduction, the 2A, 2C, 3C, and 3D proteins of EV-D68 block interferon synthesis (Xiang et al., 2014; Du et al., 2015; Lei et al., 2016; Xiang et al., 2016; Kang et al., 2021; Yang et al., 2021). More importantly, this study found that EV-D68 antagonized the antiviral effect of IFN-β by upregulating SOCS3 expression, inhibiting STAT3 phosphorylation, and blocking the transcription of antiviral ISG. It has been demonstrated that SOCS3 reduces the activity of JAK1 and JAK2 through its KIR domain and inhibits IFN-γ-driven inflammatory responses (Zhang et al., 2020). Therefore, based on our findings we can speculate that EV-D68 virus infection upregulates SOCS3 expression and inhibits JAK activity and the JAK/STAT signaling pathway through its KIR domain, which is a key means to antagonize the interferon system and inhibit EV-D68 (Fig. 6).
Fig. 6.
Schematic diagram of the mechanism of EV-D68 antagonism of the antiviral effect of IFNβ. The structural protein of EV-D68 upregulates the expression of RFX7, a transcriptional regulator of SOCS3, leading to the upregulation of SOCS3 expression. This upregulation of SOCS3 inhibits the phosphorylation of STAT3, thereby inhibiting ISG expression.
Our study shows that both inactivated EV-D68 and EV-D68 structural proteins can up-regulate SOCS3 expression, and EV-D68 upregulates SOCS3 expression by upregulating the transcriptional regulatory factor RFX7 expression. However, the mechanism by which EV-D68 upregulates SOCS3 expression could also be due to triggering pattern recognition receptor signaling early in infection. It has been reported that EV71 infection leads to the activation of the NLRP3 inflammasome (Wang et al., 2015); it has also been shown that SOCS3 expression is enhanced under inflammatory conditions and that many cytokines such as TNF-α, IL-1, IL-6, and IL-24 can induce upregulation of SOCS3 transcription and translation levels (Andoh et al., 2009; White et al., 2011). Therefore, we suggest that EV-D68 may also lead to inflammasome activation through the activation of NOD-like receptors, which in turn upregulates SOCS3 expression. In addition, whether the receptor of EV-D68 plays a role in the up-regulation of SOCS3 is also worthy of further study.
Viruses have various methods to block the JAK-STAT signaling pathway, such as blocking the binding of interferon to the interferon receptor, blocking the synthesis of JAK and STAT, blocking the phosphorylation of STAT3, and blocking the entry of p-STAT3 dimers into the nucleus. For instance, the UL26USP fragment of herpes simplex virus Type 1 (HSV-1) can competitively bind to IFNAR2, thereby blocking its interaction with JAK1 and IFNAR2. Avian leukemia virus (ALV-J) inhibits JAK2/STAT3 phosphorylation (Mo et al., 2021). Furthermore, the nonstructural proteins of porcine reproductive and respiratory syndrome virus (PRRSV), nsp1β, and nsp5, mediate the degradation of KPNA1 and STAT3, respectively (Yang and Zhang, 2017). Similarly, severe fever with thrombocytopenia syndrome (SFTS) virus (SFTSV) NSs isolates human STAT1 and STAT2 proteins into inclusion bodies (Ning et al., 2015; Yoshikawa et al., 2019). These strategies interfere with the IFN system by blocking the JAK-STAT pathway. In addition, recent studies have shown that EV71 can utilize autologous 3C protease (3Cpro) to cleave intracellular OAS3 and enhance viral replication (Zhou et al., 2022). It remains unknown whether EV-D68 employs alternative methods to block JAK-STAT signaling.
5. Conclusions
This study revealed that EV-D68 infection antagonized innate immune processes and was related to reducing STAT3 phosphorylation. Further studies revealed that the structural protein of EV-D68 contributes to viral replication by RFX7-mediated SOCS3 upregulation, JAK-STAT3 pathway blockage, and finally ISG inhibition. Notably, we found that the KIR domain of SOCS3 was important and designed a peptide that could specifically bind to the KIR domain of SOCS3—known as the SOCS3 inhibitor—which could inhibit EV-D68 replication. These results provided important insights into the pathogenesis of EV-D68 and the development of antiviral drugs.
Data availability
All the data generated during the current study are included in the manuscript.
Ethics statement
This study does not contain any studies with human or animal subjects performed by any of the authors.
Author contributions
Yuling Zhang: conceptualization, investigation, methodology, validation, formal analysis, data curation, writing - original draft. Leling Xu: conceptualization, investigation, methodology, validation, formal analysis, data curation, writing - original draft. Zhe Zhang: data curation, formal analysis, methodology, validation. Xin Su: data curation, formal analysis, methodology, validation. Zhiyun Wang: conceptualization, methodology, supervision, project administration. Tao Wang: conceptualization, methodology, supervision, project administration, funding acquisition.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32170144).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virs.2023.08.007.
Contributor Information
Zhiyun Wang, Email: zhiyun_wang@tju.edu.cn.
Tao Wang, Email: wangtaobio@tju.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
figs1.
References
- Andoh A., Shioya M., Nishida A., Bamba S., Tsujikawa T., Kim-Mitsuyama S., Fujiyama Y. Expression of IL-24, an activator of the JAK1/STAT3/SOCS3 cascade, is enhanced in inflammatory bowel disease. J. Immunol. 2009;183:687–695. doi: 10.4049/jimmunol.0804169. [DOI] [PubMed] [Google Scholar]
- Ayudhya S.S.N., Sips G.J., Bogers S., Leijten L.M.E., Laksono B.M., Smeets L.C., Bruning A., Benschop K., Wolthers K., Van Riel D., Geurtsvankessel C.H. Detection of intrathecal antibodies to diagnose enterovirus infections of the central nervous system. J. Clin. Virol. 2022;152:105190. doi: 10.1016/j.jcv.2022.105190. [DOI] [PubMed] [Google Scholar]
- Capobianchi M.R., Uleri E., Caglioti C., Dolei A. Type I IFN family members: similarity, differences and interaction. Cytokine Growth Factor Rev. 2015;26:103–111. doi: 10.1016/j.cytogfr.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J.Y., Gao S., Zhu C., Liu S.H., Li J.Y., Kang J., Wang Z.Y., Wang T. Typical stress granule proteins interact with the 3 ’ untranslated region of enterovirus D68 to inhibit viral replication. J. Virol. 2020;94:e02041-19. doi: 10.1128/JVI.02041-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croker B.A., Kiu H., Nicholson S.E. SOCS regulation of the JAK/STAT signalling pathway. Semin. Cell Dev. Biol. 2008;19:414–422. doi: 10.1016/j.semcdb.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H.W., Yin P.Q., Yang X.J., Zhang L.L., Jin Q., Zhu G.F. Enterovirus 71 2C protein inhibits NF-kappa B activation by binding to RelA(p65) Sci. Rep. 2015;5:14302. doi: 10.1038/srep14302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebada M.A., Fayed N., Alkanj S., Allah A.W. Enterovirus D-68 molecular virology, epidemiology, and treatment: an update and way forward. Infect. Disord. - Drug Targets. 2021;21:320–327. doi: 10.2174/1871526520666200715101230. [DOI] [PubMed] [Google Scholar]
- Fall A., Kenmoe S., Ebogo-Belobo J.T., Mbaga D.S., Bowo-Ngandji A., Foe-Essomba J.R., Tchatchouang S., Atsama M.A., Yengue J.F., Kenfack-Momo R., Feudjio A.F., Nka A.D., Mikangue C.a.M., Taya-Fokou J.B., Magoudjou-Pekam J.N., Noura E.A., Zemnou-Tepap C., Meta-Djomsi D., Maidadi-Foudi M., Kame-Ngasse G.I., Nyebe I., Djukouo L.G., Gounmadje L.K., Ngongang D.T., Oyono M.G., Emoh C.P.D., Tazokong H.R., Mahamat G.W., Kengne-Nde C.W., Sadeuh-Mba S.A., Dia N.W., La Rosa G., Ndip L.W., Njouom R. Global prevalence and case fatality rate of Enterovirus D68 infections, a systematic review and meta-analysis. PLoS Neglected Trop. Dis. 2022;16 doi: 10.1371/journal.pntd.0010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., Zhao H.L., Wang P., Wang J., Zou L.L. The roles of SOCS3 and STAT3 in bacterial infection and inflammatory diseases. Scand. J. Immunol. 2018;88 doi: 10.1111/sji.12727. [DOI] [PubMed] [Google Scholar]
- Harrison A.R., Moseley G.W. The dynamic interface of viruses with STATs. J. Virol. 2020;94:e00856-20. doi: 10.1128/JVI.00856-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodcroft E.B., Dyrdak R., Andres C., Egli A., Reist J., De Artola D.G.M., Alcoba-Florez J., Niesters H.G.M., Anton A., Poelman R., Reynders M., Wollants E., Neher R.A., Albert J. Evolution, geographic spreading, and demographic distribution of Enterovirus D68. PLoS Pathog. 2022;18 doi: 10.1371/journal.ppat.1010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm-Hansen C.C., Midgley S.E., Fischer T.K. Global emergence of enterovirus D68: a systematic review. Lancet Infect. Dis. 2016;16:E64–E75. doi: 10.1016/S1473-3099(15)00543-5. [DOI] [PubMed] [Google Scholar]
- Hoorn B., Tyrrell D.A. ON the growth of certain "newer" respiratory viruses in organ cultures. Br. J. Exp. Pathol. 1965;46:109–118. [PMC free article] [PubMed] [Google Scholar]
- Hsia H.C., Stopford C.M., Zhang Z.G., Damania B., Baldwin A.S. Signal transducer and activator of transcription 3 (Stat3) regulates host defense and protects mice against herpes simplex virus-1 (HSV-1) infection. J. Leukoc. Biol. 2017;101:1053–1064. doi: 10.1189/jlb.4A1016-199RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y.M., Musharrafieh R., Zheng M., Wang J. Enterovirus D68 antivirals: past, present, and future. ACS Infect. Dis. 2020;6:1572–1586. doi: 10.1021/acsinfecdis.0c00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B.Z., Chen H.P., Zheng Y.B. MiR-103/miR-107 inhibits enterovirus 71 replication and facilitates type I interferon response by regulating SOCS3/STAT3 pathway. Biotechnol. Lett. 2021;43:1357–1369. doi: 10.1007/s10529-021-03115-z. [DOI] [PubMed] [Google Scholar]
- Kang J., Pang Z., Zhou Z.W., Li X.H., Liu S.H., Cheng J.Y., Liu P.Y., Tan W.J., Wang Z.Y., Wang T. Enterovirus D68 protease 2A(pro) targets TRAF3 to subvert host innate immune responses. J. Virol. 2021;95:e01856-20. doi: 10.1128/JVI.01856-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.G.M., Ghosh A., Variya B., Santharam M.A., Kandhi R., Ramanathan S., Ilangumaran S. Cytokine; 2019. Hepatocyte Growth Control by SOCS1 and SOCS3; p. 121. [DOI] [PubMed] [Google Scholar]
- Kohil A., Jemmieh S., Smatti M.K., Yassine H.M. Viral meningitis: an overview. Arch. Virol. 2021;166:335–345. doi: 10.1007/s00705-020-04891-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X.B., Xiao X., Wang J.W. vol. 8. Viruses-Basel; 2016. (Innate Immunity Evasion by Enteroviruses: Insights into Virus-Host Interaction). 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linossi E.M., Calleja D.J., Nicholson S.E. Understanding SOCS protein specificity. Growth Factors. 2018;36:104–117. doi: 10.1080/08977194.2018.1518324. [DOI] [PubMed] [Google Scholar]
- Liongue C., O’sullivan L.A., Trengove M.C., Ward A.C. Evolution of JAK-STAT pathway components: mechanisms and role in immune system development. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo G.D., Fu H.L., Hu B.W., Zhang Q.H., Xian M.J., Zhang Z.H., Lin L., Shi M.Q., Nie Q.H., Zhang X.Q. SOCS3 promotes ALV-J virus replication via inhibiting JAK2/STAT3 phosphorylation during infection. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.748795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P.J. The JAK-STAT signaling pathway: input and output intergration. J. Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- Negishi H., Taniguchi T., Yanai H. The interferon (IFN) class of cytokines and the IFN regulatory factor (IRF) transcription factor family. Cold Spring Harbor Perspect. Biol. 2018;10:a028423. doi: 10.1101/cshperspect.a028423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning Y.J., Feng K., Min Y.Q., Cao W.C., Wang M.L., Deng F., Hu Z.H., Wang H.L. Disruption of type I interferon signaling by the nonstructural protein of severe fever with thrombocytopenia syndrome virus via the hijacking of STAT2 and STAT1 into inclusion bodies. J. Virol. 2015;89:4227–4236. doi: 10.1128/JVI.00154-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste M.S., Maher K., Schnurr D., Flemister M.R., Lovchik J.C., Peters H., Sessions W., Kirk C., Chatterjee N., Fuller S., Hanauer J.M., Pallansch M.A. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. J. Gen. Virol. 2004;85:2577–2584. doi: 10.1099/vir.0.79925-0. [DOI] [PubMed] [Google Scholar]
- Opanda S.M., Wamunyokoli F., Khamadi S., Coldren R., Bulimo W.D. Genetic diversity of human enterovirus 68 strains isolated in Kenya using the hypervariable 3 ’-End of VP1 gene. PLoS One. 2014;9 doi: 10.1371/journal.pone.0102866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieble J.H., Fox V.L., Lennette E.H. A probable new human picornavirus associated with respiratory diseases. Am. J. Epidemiol. 1967;85:297–310. doi: 10.1093/oxfordjournals.aje.a120693. [DOI] [PubMed] [Google Scholar]
- Skowronski D.M., Chambers C., Sabaiduc S., Murti M., Gustafson R., Pollock S., Hoyano D., Rempel S., Allison S., De Serres G., Dickinson J.A., Tellier R., Fonseca K., Drews S.J., Martineau C., Reyes-Domingo F., Wong T., Tang P., Krajden M. Systematic community- and hospital-based surveillance for enterovirus-D68 in three Canadian provinces, August to December 2014. Euro Surveill. 2015;20:11–24. doi: 10.2807/1560-7917.ES.2015.20.43.30047. [DOI] [PubMed] [Google Scholar]
- Smura T., Blomqvist S., Paananen A., Vuorinen T., Sobotova Z., Bubovica V., Ivanova O., Hovi T., Roivainen M. Enterovirus surveillance reveals proposed new serotypes and provides new insight into enterovirus 5'-untranslated region evolution. J. Gen. Virol. 2007;88:2520–2526. doi: 10.1099/vir.0.82866-0. [DOI] [PubMed] [Google Scholar]
- Waiboci L.W., Ahmed C.M., Mujtaba M.G., Flowers L.O., Martin J.P., Haider M.I., Johnson H.M. Both the suppressor of cytokine signaling 1 (SOCS-1) kinase inhibitory region and SOCS-1 mimetic bind to JAK2 autophosphorylation site: implications for the development of a SOCS-1 antagonist. J. Immunol. 2007;178:5058–5068. doi: 10.4049/jimmunol.178.8.5058. [DOI] [PubMed] [Google Scholar]
- Wang H.B., Lei X.B., Xiao X., Yang C.F., Lu W.L., Huang Z., Leng Q.B., Jin Q., He B., Meng G.X., Wang J.W. Reciprocal regulation between enterovirus 71 and the NLRP3 inflammasome. Cell Rep. 2015;12:42–48. doi: 10.1016/j.celrep.2015.05.047. [DOI] [PubMed] [Google Scholar]
- Wang X.Z., Wen L., Zhou Y.P., Huang S.N., Yang B., Cheng S., Zeng W.B., Mei M.J., Sun J.Y., Jiang X., Cheng H., Luo M.H. Human cytomegalovirus pUL97 upregulates SOCS3 expression via transcription factor RFX7 in neural progenitor cells. PLoS Pathog. 2023;19 doi: 10.1371/journal.ppat.1011166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W., Guo H.R., Chang J.L., Yu Y.Z., Liu G.C., Zhang N.N., Willard S.H., Zheng S., Yu X.F. ICAM-5/Telencephalin is a functional entry receptor for enterovirus D68. Cell Host Microbe. 2016;20:631–641. doi: 10.1016/j.chom.2016.09.013. [DOI] [PubMed] [Google Scholar]
- Wei Y.R., Zhang Z.Y., She N., Chen X., Zhao Y., Zhang J.L. Atomistic insight into the inhibition mechanisms of suppressors of cytokine signaling on Janus kinase. Phys. Chem. Chem. Phys. 2019;21:12905–12915. doi: 10.1039/c9cp02257k. [DOI] [PubMed] [Google Scholar]
- White G.E., Cotterill A., Addley M.R., Soilleux E.J., Greaves D.R. Suppressor of cytokine signalling protein SOCS3 expression is increased at sites of acute and chronic inflammation. J. Mol. Histol. 2011;42:137–151. doi: 10.1007/s10735-011-9317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z.C., Li L.L., Lei X.B., Zhou H.L., Zhou Z., He B., Wang J.W. Enterovirus 68 3C protease cleaves TRIF to attenuate antiviral responses mediated by toll-like receptor 3. J. Virol. 2014;88:6650–6659. doi: 10.1128/JVI.03138-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z.C., Liu L.L., Lei X.B., Zhou Z., He B., Wang J.W. 3C protease of enterovirus D68 inhibits cellular defense mediated by interferon regulatory factor 7. J. Virol. 2016;90:1613–1621. doi: 10.1128/JVI.02395-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J.Y., Wang M.S., Cheng A.C., Jia R.Y., Zhu D.K., Liu M.F., Chen S., Zhao X.X., Yang Q., Wu Y., Zhang S.Q., Luo Q.H., Wang Y., Xu Z.W., Chen Z.L., Zhu L., Liu Y.Y., Yu Y.L., Zhang L., Chen X.Y. The role of SOCS proteins in the development of virus- induced hepatocellular carcinoma. Virol. J. 2021;18:74. doi: 10.1186/s12985-021-01544-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F.W., Zhao X.X., Hu S.Q., Li J., Yin L.J., Mei S., Liu T.T., Wang Y., Ren L.L., Cen S., Zhao Z.D., Wang J.W., Jin Q., Liang C., Ai B., Guo F. Amphotericin B inhibits enterovirus 71 replication by impeding viral entry. Sci. Rep. 2016;6:33150. doi: 10.1038/srep33150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.P., Zhang Y.J. Antagonizing cytokine-mediated JAK-STAT signaling by porcine reproductive and respiratory syndrome virus. Vet. Microbiol. 2017;209:57–65. doi: 10.1016/j.vetmic.2016.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.N., Zheng H.W., Li H., Chen Y.L., Hou D.P., Fan Q.Q., Song J., Guo L., Liu L.D. The expression of IFN-beta is suppressed by the viral 3D polymerase via its impact on PGAM5 expression during enterovirus D68 infection. Virus Res. 2021;304:198549. doi: 10.1016/j.virusres.2021.198549. [DOI] [PubMed] [Google Scholar]
- Yokota S., Yokosawa N., Okabayashi T., Suzutani T., Miura S., Jimbow K., Fujii N. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 contributes to inhibition of the interferon signaling pathway. J. Virol. 2004;78:6282–6286. doi: 10.1128/JVI.78.12.6282-6286.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa R., Sakabe S., Urata S., Yasuda J. Species-specific pathogenicity of severe fever with thrombocytopenia syndrome virus is determined by anti-STAT2 activity of NSs. J. Virol. 2019;93:e02226-18. doi: 10.1128/JVI.02226-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.J., He B.Q., Li H., Wang Y.J., Zhou Y., Wang W.J., Song T.C., Du N., Gu X.X., Luo Y., Wang Y.J. SOCS3 attenuates GM-CSF/IFN-gamma-Mediated inflammation during spontaneous spinal cord regeneration. Neurosci. Bull. 2020;36:778–792. doi: 10.1007/s12264-020-00493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.L., Tian L., Wang J., Zheng B.S., Zhang W.Y. EV71 3C protease cleaves host anti-viral factor OAS3 and enhances virus replication. Virol. Sin. 2022;37:418–426. doi: 10.1016/j.virs.2022.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data generated during the current study are included in the manuscript.