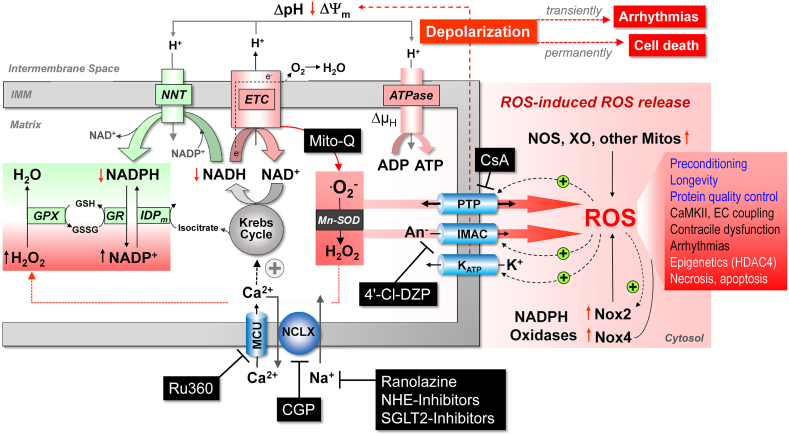
Fig. 11.
Regulation of mitochondrial respiration and redox state by ion handling. The Krebs cycle is stimulated by Ca2+ that enters mitochondria via the mitochondrial Ca2+ uniporter (MCU) and is exported by the mitochondrial Na+/Ca2+-exchanger (NCLX). The Krebs cycle produces NADH, which donates electrons to the electron transport chain (ETC). Sequential redox reactions along the ETC establish a proton gradient (ΔpH) across the inner mitochondrial membrane (IMM) which together with the electrical potential (ΔΨm) constitutes the proton motive force (ΔμH), which is harnessed by the F1/Fo-ATP synthase (ATPase) to regenerate ATP via oxidative phosphorylation of ADP. During respiration, superoxide (O2−) is generated at complexes I and III, which are dismutated to hydrogen peroxide (H2O2) by the Mn2+-dependent superoxide dismutase (MnSOD). H2O2 is then eliminated by glutathione peroxidase (GPX) and the thioredoxin/peroxiredoxin system (not shown). GPX is regenerated by reduced glutathione (GSH), which in turn is reduced by the glutathione reductase (GR), which uses NADPH that is produced by NADP+-dependent isocitrate dehydrogenase (IDPm) and the nicotinamide nucleotide transhydrogenase (NNT). Reactive oxygen species (ROS) from NADPH oxidases (Nox) 2 and 4, but also xanthine/xanthine oxidase (XO), nitric oxide synthase (NOS) or other mitochondria (Mitos) can activate redox-sensitive ion channels in the IMM, such as the permeability transition pore (PTP), the inner mitochondrial membrane anion channel (IMAC) or the ATP-sensitive K+-channel (KATP). Opening of these channels dissipates ΔΨm, requiring accelerated electron flux along the ETC to maintain ΔΨm. This oxidizes NADH and (via reverse-mode NNT) NADPH and thereby, the antioxidative capacity, limiting H2O2 elimination. ROS can leave mitochondria through the IMAC or PTP and trigger ROS release from neighboring mitochondria. Depending on the concentrations and durations of ROS elevations, ROS can serve protective roles, such as ischemic preconditioning, longevity and/or protein quality control, but at higher concentrations can deteriorate excitation-contraction coupling and induce epigenetic signaling, apoptosis and/or necrosis. When ΔΨm (transiently or permanently) dissipates, ATP production ceases, which activates sarcolemmal KATP channels, making the cell inexcitable. Heterogeneities of ΔΨm in different cardiac myocytes within the myocardium resemble “metabolic sinks” which can induce re-entry mechanisms to induce arrhythmias. In heart failure, elevated cytosolic [Na+]i accelerates mitochondrial Ca2+ extrusion, which can be ameliorated by inhibiting the NCLX with CGP-37157 (CGP) or lowering [Na+]i by inhibitors of the Na+/H+-exchanger (NHE), of late Na+ current (i.e., ranolazine) and as observed for Sodium/Glucose Co-transporter 2 (SGLT2)-inhibitors via inhibiting NHE and/or late Na+ current. CsA, cyclosporine A. GSSG, oxidized glutathione. CaMKII, Ca2+/calmodulin-dependent protein kinase II; HDAC4, histone deacetylase 4; EC coupling, excitation-contraction coupling. Modified from [398].