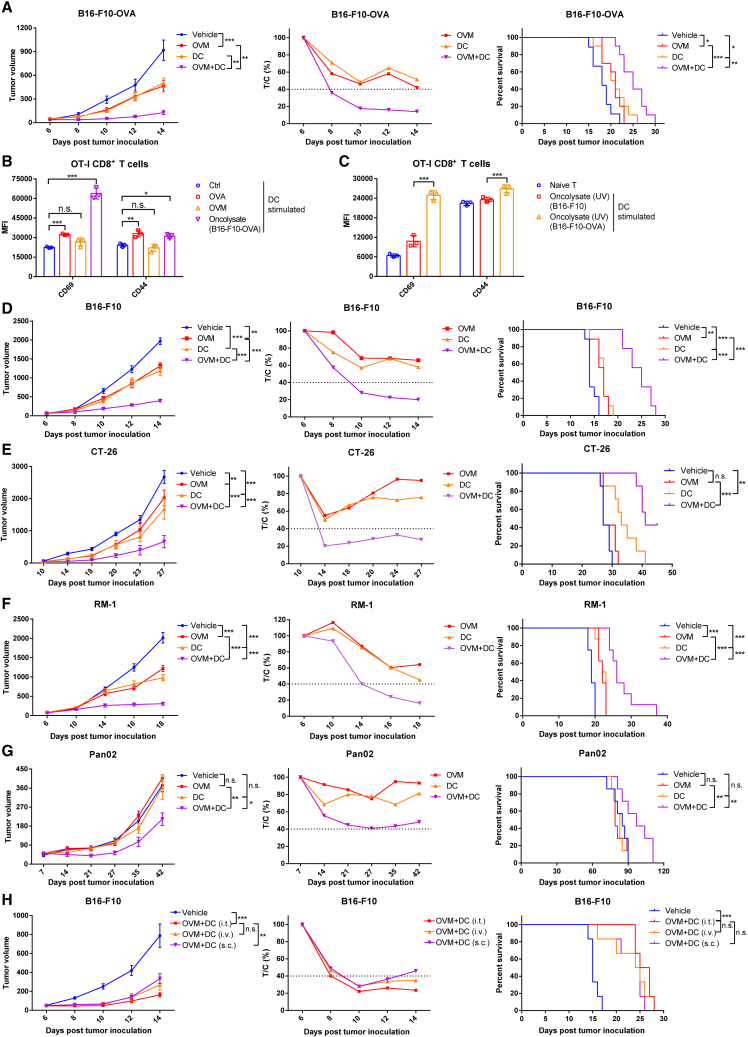
Figure 1.
OVM enhances the therapeutic efficacy of a DC vaccine
(A) C57BL/6J mice were implanted subcutaneously in the right flank with B16-F10-OVA cells and treated with vehicle (n = 9), OVM (n = 10), DC vaccine (stimulated by OVA and LPS) (n = 10), or OVM plus DC vaccine (n = 10) (tumor volumes were approximately 50 mm3). OVM was administered via tail vein injection for 5 consecutive days. And DC vaccine was administered through intratumoral injection once. Left: tumor growth curves. Center: the relative tumor proliferation rate (T/C %). Right: Kaplan-Meier survival curves.
(B) The mean fluorescence intensity (MFI) values of the activation markers CD69 and CD44 on the OT-I CD8+ T cells stimulated by differently treated DCs. DCs were subjected to one of the following treatments for 24 h: control (Ctrl), OVA (1 μg/mL, as positive control), OVM (1 MOI), or B16-F10-OVA oncolysate (1 mL). After treatment, the DCs were washed with PBS and cocultured with spleen-derived CD8+ T cells from OT-I mice at a 1:2 ratio for 48 h; n = 3.
(C) The MFI of CD69 and CD44 on the OT-I CD8+ T cells was assessed after stimulation by DCs at a 1:2 ratio for 48 h. These DCs were treated with UV-inactivated B16-F10-OVA oncolysate (1 mL) or UV-inactivated B16-F10 oncolysate (1 mL) for 24 h; n = 3.
(D–G) C57BL/6J or BALB/c mice were implanted subcutaneously in the right flank with B16-F10 (n = 9), CT-26 (n = 7), RM-1 (n = 8), or Pan02 (n = 7) cells and treated with vehicle, OVM, oncolysate-activated DC vaccine, or OVM combined with DC vaccine. OVM was administered via tail vein injection for 5 consecutive days. And DC vaccine was administered through intratumoral injection twice. Tumor growth curves (left), T/C % (center), and Kaplan-Meier survival curves (right) of B16-F10 (D), CT-26 (E), RM-1 (F), and Pan02 (G) tumor-bearing mouse models.
(H) C57BL/6J mice were implanted subcutaneously in the right flank with B16-F10 cells and treated with vehicle (n = 6) or OVM combined with B16-F10 oncolysate-activated DC vaccine (n = 6). OVM was administered via tail vein injection for 5 consecutive days. And DC vaccine was administered twice using different routes, including tail vein injection, intratumoral injection, or subcutaneous injection near the tumor site. Tumor growth curves (left), T/C % (center), and Kaplan-Meier survival curves (right) are shown. The p values were determined by one-way ANOVA or by the log rank test. n.s., not significant; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.