Abstract
The cell surface antigen CD14 is primarily understood to act as a co-receptor for toll-like receptors (TLRs) to activate innate immunity responses to pathogens and tissue injury in macrophages and monocytes. However, roles for CD14 are increasingly being uncovered in disease responses in epithelial and endothelial cells. Consistent with these broader functions, CD14 expression is altered in a variety of non-immune cell types in response to a several of disease states. Moreover, soluble CD14 activated by factors from both pathogens and tissue damage may initiate signaling in a variety of non-immune cells. This review examined the current understanding CD14 in innate immunity as well as its potential functions in nonimmune cells and associated human diseases.
Keywords: CD14, Immunity, Organ injury, Inflammation, LPS, Metabolism, Human disease
Introduction:
Canonically, the CD14 glycoprotein receptor has been shown to promote macrophage activation upon binding either LPS or oxidized lipids. CD14 was initially recognized as a membrane receptor for lipopolysaccharides (LPS) on macrophages and to mediate their host cytokine response to sepsis, most notably TNF [1]. In addition to macrophages, CD 14 has expression has been detected in a variety of other immune cell types including human and mouse neutrophils and dendritic cells [2] and at lower levels in human T-cells and B-cells and liver resident Kupffer cells [3–6]. In the non-immune compartment, CD14 expression was also detected in human enterocytes [7] human and mouse hepatocytes [8, 9] and pancreatic islet beta cells [10]. CD14 has also been found to bind other pathogen-associated molecular pattern (PAMP) type ligands such as Lipoteichoic acid (LTA) and Biglycan [11, 12]. In addition, CD14 participates in the inflammatory responses by binding oxidized lipids with damage-associated molecular patterns (DAMPs) that accumulate following organ injury, autoimmune disorders and atherosclerosis [13, 14]. After binding to either PAMPs or DAMPs in response to infection or organ injury, CD14 initiates signaling involved in the early activation phase of the innate immune system. During both acute and chronic innate immune activation, CD14 dependent signaling also regulates metabolic processes. Of note, the soluble form of CD14 enhances the LPS response in non-inflammatory cells, implicating it in functions beyond innate immunity [14, 15]. Indeed, soluble CD14 originating from the liver was identified as an acute phase response protein in chronic inflammatory disease [16]. This review relates the role of CD14 in human diseases to potential novel functions that may contribute to disease pathologies.
CD14 Structure:
CD14 is biologically active as either a monomeric protein [17] attached to the cell membrane via a glycosylphosphatidylinositol (GPI) anchor (mCD14) or as a secreted soluble protein (sCD14) that enables epithelial and endothelial cells to respond to LPS [18]. Known CD14 ligands, including PAMPs and DAMPs are listed in Table 1. In human glioblastoma cells, the crystal structure of CD14 protein revealed a bent solenoid structure containing thirteen strands, eleven of which overlap with leucine-rich repeat regions (LRRs). Eleven parallel and two antiparallel strands create a concave surface, where disulfide bonds and N-linked glycosylation near the n-terminal strands are crucial for proper folding and signaling [19]. The ligand binding pocket at the N-terminus of CD14 consists of hydrophobic residues in , with charged residues around the pocket rim. The charged residues have been proposed to orient the binding of the phosphate groups attached to the core oligosaccharide of LPS. Ligand binding to soluble murine CD14 occurs at four regions: 26DEES29, lying between the and strands, 37PKPD40 in the loop between the strand at 50AADVE54 in the helix and the strand, and at 73ADLGQF78 in the loop between the and helices [20]. While the human CD14 binding pocket cannot accommodate all the acyl chains of a ligand, these chains may bind to additional grooves at the C-terminal side of the pocket and to the flexible hydrophilic rim. These adjacent residues also participate in activating intracellular signaling by the target cell. The bent solenoid structures of mouse and human CD14 are highly superimposable (root mean square deviation: 1.089 Å). However, differences exist in charged residues on the rim and between the shape of the N-terminal pocket. For instance, a positively charged K38 residue in murine CD14 is represented by a negatively charged D44 residue at this position in human CD14. Further, hydrophobic interactions between residues F45, F78, L49, and I81 in mouse CD14 close off one side of the pocket, while corresponding T85 and L89 residues in human CD14 allow the extension of the pocket diameter [20, 21]. However, both human and mouse CD14 likely bind similar ligands to activate concordant cellular pathways [6].
Table1:
List of some PAMPs and DAMPs recognized by CD14.
CD14 binding to LPS:
The first discovered and most studied ligand of CD14 is the endotoxin lipopolysaccharide (LPS). The outer membrane of gram-negative bacteria consists of LPS, lipid A, a core oligosaccharide, and an O-antigen chain of variable length [22]. In serum, soluble CD14 recognizes various LPS chemotypes in complex with LPS-binding protein (LBP). This includes “rough” colonies that have truncated versions of the O-antigen chain or “smooth” colonies that preserve the O-antigen chain [23]. Macrophages from wild-type CD14+/+ mice recognize smooth LPS at lower picomolar concentrations than rough LPS. Whereas, CD14−/− macrophages demonstrate no significant preference for either smooth or rough LPS but have a 150,000 fold reduction in overall sensitivity to LPS induced TNF- production [24]. These data strongly demonstrate a critical role for CD14 in the response of macrophages to the smooth LPS ligand.
CD14 binding to toll-like receptors:
Toll-like receptors (TLRs) function in the innate immune system as pattern-recognition receptors (PRRs) to identify pathogen-associated molecular patterns (PAMPs) on bacteria and viruses [25]. Upon such engagement, TLRs initiate pro-inflammatory signaling that activates transcription factors such as NF-B and IRFs in dendritic cells to initiate adaptive immunity. In addition, primarily TLR4 responds to accessory molecules on the plasma membrane, such as MD-2 and RP105, and the endoplasmic reticulum, such as PRAT4A and Gp96. CD14 acts a co-receptor in this process by directly binding to TLRs and by transferring LPS to the TLR-MD-2 complex. CD14 further promotes TLRs to engage with adapter proteins, such as MyD88, Mal (TIRAP), TRIF, and TRAM, to initiate intracellular signaling [25].
Signaling Pathways involving CD14:
Nuclear Receptor in Activated T Cell (NFAT) Signaling
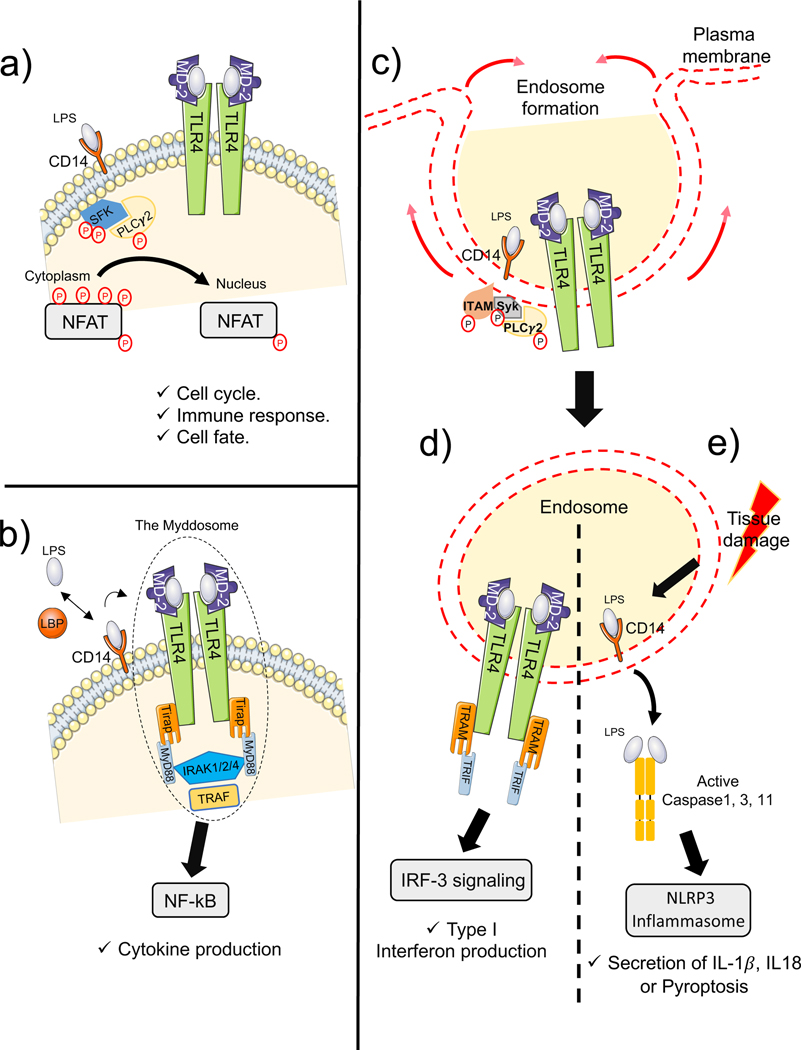
Engagement of the CD14-LPS complex initiates TLR4-independent NFAT activation in a variety of inflammatory cell types. In human and murine dendritic cells, upon binding LPS, CD14 localizes to lipid rafts and directly recruits src family kinase (SFK) and phospholipase C2 (PLC2) [26, 27]. Subsequent Ca2+ influx activates the calcineurin phosphatase which dephosphorylates NFAT to promote its translocation into the nucleus where it drives pro-apoptotic gene expression that enables self-tolerance (Figure1a) [28]. Due to its GPI anchor, the direct role of CD14 in Ca2+ mobilization has been questioned. An alternate model posits that Ca2+ influx is primarily initiated by agonization of the Fc receptor gamma chain receptor (FcR) and DAP12 signaling adaptor which results in phosphorylation of its immunoreceptor tyrosine-based activation motif (ITAM) motif and recruitment of SYK that in turn activates Phospholipase C gamma (PLC) [29].
Figure1: CD14 activates multiple signaling pathways.
a) In both human and mouse dendritic cells, CD14-LPS complex initiates TLR4-independent NFAT activation in a variety of inflammatory cell types. CD14 localizes to lipid rafts and directly recruits Src family kinase (SFK) and phospholipase C2 (PLC2). Subsequent dephosphorylation of NFAT promotes its translocation into the nucleus where it drives pro-apoptotic gene expression, thus enabling self-tolerance. b) LBP-dependent combination of the CD14-LPS complex with TLR4-MD-2 in lipid rafts promotes TIRAP-MyD88 to bind to the TIR domains of TLR4. The newly formed “myddosome” triggers the activation of NF-B and MAPK through the TNF-receptor associated factor (TRAF). Consequently, numerous proinflammatory cytokines such as TNF-, IL-1, and IL-6 are secreted. c) LPS bound CD14 activates ITAM-containing adaptor proteins that stimulate the tyrosine kinase Syk and PLC2 to initiate the delivery of the TLR4/MD2 complex from the plasma membrane to endosomal compartments. d) In endosomal vesicles, CD14 further enables LPS to stimulate the TRAM-TRIF pathway to illicit interferon-3 regulatory factor (IRF3) production and subsequent type-1 IFN production. e) In human monocytes, epithelial cells and keratinocytes, when CD14 binds to LPS during tissue damage, its endocytosis allows it to bind and activate the inflammasome assembly independently of TLRs. CD14 activates the NLRP3-mediated inflammasome assembly that results in the release of IL-1 and Il-18 with or without cell death.
MyD88-TIRAP Signaling
In murine macrophages, association of the CD14-LPS complex with TLR4-MD-2 in lipid rafts promotes TIRAP-MyD88 to bind to the TIR domains of TLR4 (Figure 1b). For non-immune cells that express inadequate levels of mCD14, LPS is transferred to MD2 by circulating sCD14 resulting in TLR pathway activation [30, 31]. Such activation involves MyD88 recruitment of IL-1R-associated kinase-4 (IRAK4) and IRAK2/1 [32] resulting in assembly of the myddosome; a large oligomeric signaling complex that elicits an inflammatory immune response in the innate host response to infection. The myddosome triggers activation of NF-B and MAPK by activating the TNF-receptor associated factor (TRAF) [33]. Consequently, the proinflammatory cytokines TNF-, IL-1, and IL-6 are secreted. Such signaling was also described in endothelial cells [34] and neutrophils [35], whereas in LPS-stimulated murine mast cells, The TRAM signaling branch leading both to NF-kB activation and enhanced proinflammatory cytokine production is absent [36].
TRAM-TRIF Signaling
CD14 also regulates the microbial induced endocytosis of Toll-like Receptors (TLRs) to enable intracellular signaling that promotes innate immunity. Upon dendritic cell exposure to inflammatory mediators, CD14 activates ITAM-containing adaptor proteins that stimulate the tyrosine kinase Syk and PLC2 to initiate the delivery of the TLR4/MD2 complex from the plasma membrane to endosomal compartments [37]. In vesicles, CD14 further enables LPS to stimulate the TRAM-TRIF pathway to illicit interferon-3 regulatory factor (IRF3) production and subsequent type-1 IRF production (Figure1c,d) [38]. However, the activation of TRAM-TRIF can be CD14-independent if LPS is delivered to endosomal compartments via microbeads or liposome combinations [39].
Inflammasome Signaling through a non-TLR pathway
In the airway epithelium, CD14+ macrophages and dendritic cells recognize signatures associated with cell death to promote inflammasome assembly, independent of TLRs. When CD14 is bound to LPS or oxidized lipids (Oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine, aka oxPAPC) during tissue damage, its endocytosis allows it to directly promote inflammasome assembly [40]. OxPAPCs function as damage-associated molecular patterns (DAMP) that induce CD14 to activate NLRP3 mediated inflammasome assembly that results in the release of IL-1 and Il-18 without cell death (Figure 1e) [41]. Whereas LPS associated CD14 activates non-canonical caspase3 containing inflammasomes that induce a specialized form of cell death termed pyroptosis [42]. In addition to its effects on inflammasomes, endocytosis of CD14 allows it to also recognize sLPS and consequently to inhibit both the MyD88-TIRAP and TRIF-TRAM pathways [42].
CD14 in human diseases:
CD14 In Infectious diseases
Many studies found a critical role for CD14 in the host response to sepsis. The levels of CD14 in serum both associates with severity and prognosis of sepsis in neonates [43]. CD14 levels also predict sepsis in burn patients [44]. In sepsis-related acute lung injury, CD14 levels are upregulated and its inhibition in animal models decreases injury [45]. In both porcine and primate models, CD14 inhibition with neutralizing antibodies associates with improved prognosis from E. Coli induced sepsis [46]. CD14 is also elevated in patient populations with a number of acute infectious processes including active tuberculosis infections [47], periodontitis [48], influenza [49], HIV, HCV [50], and covid-19 [51]. For covid-19, CD14 levels significantly predict disease severity. CD14 blockade was proposed as a potential strategy to control inflammation and organ injury due to SARS-CoV-2 infection [52]. Furthermore, methotrexate, a widely used immunosuppressive agent against psoriasis, decreases both CD14 expression and the responsiveness of macrophage to LPS [53].
CD14 Involvement in Metabolic Disease
Several studies find a role for CD14 in metabolism and insulin sensitivity. CD14 has been shown to influence adrenal function to increase glucose responsiveness [54] consistent with its observed decrease in patient serum after weight loss. Injection of recombinant human CD14 in lean and obese mice has also been found to result in sepsis independent changes in adipose cell expression of metabolic genes. Interestingly, CD14-deficient mice subjected to high-fat diet are more resistant to obesity, insulin resistance and cardiovascular disease [55]. Subsequently, CD14 has been shown to regulate SIRT1 activity, a master regulator of metabolism [56]. In addition, CD14 may modulate leptin responsiveness during the pathogenesis of steatohepatitis associated with obesity [57]. Interestingly, NFAT signaling, one of the downstream signals of CD14, was found necessary for fat accumulation in high-fat diet models [58]. More recently, the description of TLR-dependent inflammasome activation in adipose tissue of patients with cancer cachexia [59] indicates a potential role of CD14 in cancer associated cachexia. In skeletal muscle, NFAT pathway plays an important role in oxidative metabolism and fiber type switching after exposure to thymol [60]. Taken together, CD14 has a significant role in the metabolic response to both infection and other inflammatory states.
CD14 involvement in cardiovascular diseases
Elevated levels of CD14 in patients associates with higher levels of cholesterol coupled with greater risks of myocardial infarction and cardiovascular-associated death [61]. CD14 also appears to enhance the severity of cardiomyopathic conditions that result from third degree burn injury [62] and Chagas disease [63]. Finally, CD14 levels have been suggested to predict patient outcomes following acute myocardial infarction [64]. The role of CD14 in cardiovascular function has been linked to its ability to activate cytokine secretion and inflammation in macrophages by the atherosclerosis-inducing low-density lipoprotein (LDL) metabolites that are highly linked to cardiovascular disease progression. This is supported by findings that CD14 neutralization in macrophages results in the inhibition of secretion of cytokines stimulated by LDL as well as the suppression of proinflammatory cytokine activation to a greater extent than any TLR examined [65]. High levels of CD14 therefore appear to be highly causal in the genesis of atherosclerosis and associated heart disease as well as myocardial dysfunction.
CD14 and autoimmune conditions
Significant evidence indicates that CD14 promotes the pathogenesis of autoimmune diseases. CD14 levels in serum may serve as a biomarker for rheumatoid arthritis prognosis and response to methotrexate [53]. CD14 polymorphisms associated with greater CD14 expression levels are also increased in patients with systemic lupus erythematosus [66]. Whereas elevated levels of CD14 in celiac disease decline with resolution of inflammation [67]. CD14 has also been identified as a potential mediator of amyotrophic lateral sclerosis (AMLS) [68] and strategies for CD14 neutralization for AMLS treatment are being pursued. A more general role of CD14 in autoimmune conditions is supported by its elevated levels in patients with systemic sclerosis [69], psoriasis [70], Kawasaki’s disease [71], acute pancreatitis [72], osteoarthritis [49], vasculitis [73], and primary biliary cholangitis [74].
CD14 involvement in cancer
CD14 polymorphisms that result in increased CD14 expression levels are associated with increased risks of developing several malignancies [75]. High levels of CD14 in tumor associated macrophages associate with reduced anti-cancer CD8+ inflammatory T cell activity; partly through the increased secretion of the chemokine CXC12 and acetylation of p53 [56]. In patients, CD14 expression associates with more aggressive colorectal and kidney cancers and poor patient outcomes [76]. In gastric cancer, CD14 is implicated in increasing tumor invasiveness through activation of E-cadherin [77]. Whereas, CD14 levels are elevated in ovarian cancer, pulmonary non-small cell cancers [78], hepatocellular carcinoma [79], bladder cancer [80], and laryngeal cancer [81]. Conversely, analysis of 10 cancer stem cell markers revealed that CD14 marks a subpopulation of non-tumorigenic breast cancer cell line [82]. In pancreatic cancer, CD14/TLR pathways prime the tumor associated macrophages to exert anti-tumor action [83]. Despite the accumulating evidence for a role of CD14 in numerous cancers, the lack of functional and mechanistic studies may explain the lack of therapeutic approaches to target CD14 in human cancer.
CD14 gene expression changes in pathogenesis
Gene expression studies showed concordant dysregulation of CD14 expression in several human diseases (Table 2, Supplementary table 1). This suggests that signaling downstream of CD14 might play a role in many pathologies. Unfortunately, most of these gene expression studies did not provide mechanistic insights on how CD14 is leading to pathogenesis. Although, changes in CD14 expression clearly correlate with diseases, for instance, the role of CD14 in carcinogenesis and other pathological metabolic processes is still unclear.
Table 2: CD14 mRNA expression in selected human diseases organized by organ/system.
Different diseases and conditions from human and experimental animal models are shown. Species studied are shown, H: Human, M: Mouse, R: Rat. UP: Upregulated. DN: Downregulated. Data and disease scores were generated using the Base space correlation engine / disease atlas / Illumina. The Full data on the query “CD14 in disease atlas” can be found in (Supplementary Table1).
| Organ/System | Disease | CD14 mRNA | Score | References | Species |
|---|---|---|---|---|---|
| Hematopoietic | Bleeding | UP | 76 | [97] | M |
| T-cell lymphoma | UP | 72 | [98] | H, M | |
| Transplant rejection | UP | 61 | [99] | H, M | |
| Hemoglobinopathy | DN | 47 | [100] | H | |
| Genitourinary | Nephritis | UP | 76 | [101] | M |
| Renal fibrosis | UP | 63 | [102] | H | |
| Urogenital injury | UP | 55 | [103] | M, R | |
| Kidney cancer | UP | 50 | [104] | H | |
| Brain & nervous system | Brain hypoxia | UP | 74 | [105] | H, M |
| Nerve injury | UP | 73 | [106] | M, R | |
| Huntington disease | UP | 56 | [107] | H | |
| Brain cancer | UP | 48 | [108] | H | |
| Lung | Infectious disease of lung | UP | 74 | [109] | M |
| Pneumonia | UP | 64 | [110] | H, R | |
| Digestive system | Intestinal ulceration | DN | 70 | [111] | H |
| Intestinal infectious disease | UP | 51 | [112] | H, M | |
| Inflammatory bowel disease | UP | 47 | [112] | H | |
| Gastric cancer | UP | 41 | [113] | H | |
| Musculoskeletal system | Duchenne muscular dystrophy | UP | 75 | [114] | H |
| Myopathy | UP | 52 | [115] | H | |
| Muscle atrophy | UP | 46 | [116] | H, M | |
| Liver | Injury of liver | UP | 85 | [117] | M, R |
| Liver cancer | UP | 57 | [118] | H | |
| Inflammatory disease of liver | UP | 54 | [119] | H, M | |
| Hepatic fibrosis | UP | 39 | [120] | H, M | |
| Vascular system | Shock | UP | 77 | [121] | H, M |
| Cardiomyopathy | DN | 71 | [122] | H | |
| Ischemia | UP | 67 | [123] | M, R |
Integrating our understanding of the in vivo role of CD14:
Eicke Latz has described CD14 as a factor that “shapes the immune response” [84]. Consistently, a large body of research finds that CD14 acts as a critical early mediator of the innate immune response to sepsis, organ injury and a variety of chronic inflammatory diseases such as atherosclerosis and cancer. Beyond its described expression and function in immune cells, the detection of CD14 mRNA and protein in non-immune cells such as hepatocytes, hepatic stellate cells, breast granular cells, proximal enterocytes and Langerhans cells in the skin (https://www.proteinatlas.org/ENSG00000170458-CD14/single+cell+type) suggests CD14 involvement in other physiological processes, including liver metabolism and regeneration [85, 86]. Consistently, soluble CD14 acts on many cell types including endothelial and epithelial cells. Evidence is mounting that this may represent a significant part of the action of CD14. This in part could be due to the predicted ability of the large hydrophobic pocket of CD14 to accommodate interactions with a wide variety of ligands in addition to the CD14/LPS interactions widely studied in the immune context [21]. For instance, the interaction of CD14 with oxidated phospholipids has been shown recently to promote the clearing of dead cells [13]. Furthermore, high-affinity binding of CD14 to biglycan initiates macrophage activation in response to biglycan accumulation [12]. Future studies aiming to modulate CD14 expression in chronic diseases will therefore benefit from considering potential non-canonical actions of CD14 in a variety of conditions.
Supplementary Material
Supplementary table1: Full data table showing the pathologies, disease scores and studies associated with CD14 expressaion, generated using the base space correlation engine / disease atlas / Illumina.
Funding:
This work was supported by the National Institute of Health, National Institute of General Medical Sciences: R01 GM137656/GM/NIGMS.
Footnotes
Conflict of interest:
The authors declare that this review was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References:
- 1.Wright SD, et al. , CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science, 1990. 249(4975): p. 1431–3. [DOI] [PubMed] [Google Scholar]
- 2.Setoguchi M, et al. , Mouse and human CD14 (myeloid cell-specific leucine-rich glycoprotein) primary structure deduced from cDNA clones. Biochim Biophys Acta, 1989. 1008(2): p. 213–22. [DOI] [PubMed] [Google Scholar]
- 3.Burel JG, et al. , Circulating T cell-monocyte complexes are markers of immune perturbations. Elife, 2019. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziegler-Heitbrock HW, et al. , CD14 is expressed and functional in human B cells. Eur J Immunol, 1994. 24(8): p. 1937–40. [DOI] [PubMed] [Google Scholar]
- 5.Labeta MO, et al. , Human B cells express membrane-bound and soluble forms of the CD14 myeloid antigen. Mol Immunol, 1991. 28(1–2): p. 115–22. [DOI] [PubMed] [Google Scholar]
- 6.Su GL, et al. , Activation of human and mouse Kupffer cells by lipopolysaccharide is mediated by CD14. Am J Physiol Gastrointest Liver Physiol, 2002. 283(3): p. G640–5. [DOI] [PubMed] [Google Scholar]
- 7.Funda DP, et al. , CD14 is expressed and released as soluble CD14 by human intestinal epithelial cells in vitro: lipopolysaccharide activation of epithelial cells revisited. Infect Immun, 2001. 69(6): p. 3772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su GL, et al. , CD14 expression and production by human hepatocytes. J Hepatol, 1999. 31(3): p. 435–42. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, et al. , Expression of CD14 by hepatocytes: upregulation by cytokines during endotoxemia. Infect Immun, 1998. 66(11): p. 5089–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vives-Pi M, et al. , Evidence of expression of endotoxin receptors CD14, toll-like receptors TLR4 and TLR2 and associated molecule MD-2 and of sensitivity to endotoxin (LPS) in islet beta cells. Clin Exp Immunol, 2003. 133(2): p. 208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroder NW, et al. , Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem, 2003. 278(18): p. 15587–94. [DOI] [PubMed] [Google Scholar]
- 12.Roedig H, et al. , Biglycan is a new high-affinity ligand for CD14 in macrophages. Matrix Biol, 2019. 77: p. 4–22. [DOI] [PubMed] [Google Scholar]
- 13.Zanoni I, et al. , By Capturing Inflammatory Lipids Released from Dying Cells, the Receptor CD14 Induces Inflammasome-Dependent Phagocyte Hyperactivation. Immunity, 2017. 47(4): p. 697–709 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furnkranz A, et al. , Oxidized phospholipids trigger atherogenic inflammation in murine arteries. Arterioscler Thromb Vasc Biol, 2005. 25(3): p. 633–8. [DOI] [PubMed] [Google Scholar]
- 15.Pahwa R, Devaraj S, and Jialal I, The effect of the accessory proteins, soluble CD14 and lipopolysaccharide-binding protein on Toll-like receptor 4 activity in human monocytes and adipocytes. Int J Obes (Lond), 2016. 40(6): p. 907–11. [DOI] [PubMed] [Google Scholar]
- 16.Bas S, et al. , CD14 is an acute-phase protein. J Immunol, 2004. 172(7): p. 4470–9. [DOI] [PubMed] [Google Scholar]
- 17.Leturcq DJ, et al. , Antibodies against CD14 protect primates from endotoxin-induced shock. J Clin Invest, 1996. 98(7): p. 1533–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haziot A, et al. , The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol, 1988. 141(2): p. 547–52. [PubMed] [Google Scholar]
- 19.Meng J, et al. , The differential impact of disulfide bonds and N-linked glycosylation on the stability and function of CD14. J Biol Chem, 2008. 283(6): p. 3376–3384. [DOI] [PubMed] [Google Scholar]
- 20.Kim JI, et al. , Crystal structure of CD14 and its implications for lipopolysaccharide signaling. J Biol Chem, 2005. 280(12): p. 11347–51. [DOI] [PubMed] [Google Scholar]
- 21.Kelley SL, et al. , The crystal structure of human soluble CD14 reveals a bent solenoid with a hydrophobic amino-terminal pocket. J Immunol, 2013. 190(3): p. 1304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raetz CR and Whitfield C, Lipopolysaccharide endotoxins. Annu Rev Biochem, 2002. 71: p. 635–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly NM, Young L, and Cross AS, Differential induction of tumor necrosis factor by bacteria expressing rough and smooth lipopolysaccharide phenotypes. Infect Immun, 1991. 59(12): p. 4491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gangloff SC, et al. , Influence of CD14 on ligand interactions between lipopolysaccharide and its receptor complex. J Immunol, 2005. 175(6): p. 3940–5. [DOI] [PubMed] [Google Scholar]
- 25.Zanoni I. and Granucci F, Role of CD14 in host protection against infections and in metabolism regulation. Front Cell Infect Microbiol, 2013. 3: p. 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki KG, et al. , Dynamic recruitment of phospholipase C gamma at transiently immobilized GPI-anchored receptor clusters induces IP3-Ca2+ signaling: single-molecule tracking study 2. J Cell Biol, 2007. 177(4): p. 731–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vukcevic M, et al. , Frequent calcium oscillations lead to NFAT activation in human immature dendritic cells. J Biol Chem, 2010. 285(21): p. 16003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zanoni I, et al. , CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature, 2009. 460(7252): p. 264–8. [DOI] [PubMed] [Google Scholar]
- 29.Ivashkiv LB, A signal-switch hypothesis for cross-regulation of cytokine and TLR signalling pathways. Nat Rev Immunol, 2008. 8(10): p. 816–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wurfel MM, Hailman E, and Wright SD, Soluble CD14 acts as a shuttle in the neutralization of lipopolysaccharide (LPS) by LPS-binding protein and reconstituted high density lipoprotein. J Exp Med, 1995. 181(5): p. 1743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Z, et al. , CD14 is required for MyD88-independent LPS signaling. Nat Immunol, 2005. 6(6): p. 565–70. [DOI] [PubMed] [Google Scholar]
- 32.Kawagoe T, et al. , Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat Immunol, 2008. 9(6): p. 684–91. [DOI] [PubMed] [Google Scholar]
- 33.Akira S. and Takeda K, Toll-like receptor signalling. Nat Rev Immunol, 2004. 4(7): p. 499–511. [DOI] [PubMed] [Google Scholar]
- 34.Bannerman DD, et al. , TIRAP mediates endotoxin-induced NF-kappaB activation and apoptosis in endothelial cells. Biochem Biophys Res Commun, 2002. 295(1): p. 157–62. [DOI] [PubMed] [Google Scholar]
- 35.Haziot A, Tsuberi BZ, and Goyert SM, Neutrophil CD14: biochemical properties and role in the secretion of tumor necrosis factor-alpha in response to lipopolysaccharide. J Immunol, 1993. 150(12): p. 5556–65. [PubMed] [Google Scholar]
- 36.Keck S, et al. , Absence of TRIF signaling in lipopolysaccharide-stimulated murine mast cells. J Immunol, 2011. 186(9): p. 5478–88. [DOI] [PubMed] [Google Scholar]
- 37.Zanoni I, et al. , CD14 controls the LPS-induced endocytosis of Toll-like receptor 4. Cell, 2011. 147(4): p. 868–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanimura N, et al. , Roles for LPS-dependent interaction and relocation of TLR4 and TRAM in TRIF-signaling. Biochem Biophys Res Commun, 2008. 368(1): p. 94–9. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe S, Kumazawa Y, and Inoue J, Liposomal lipopolysaccharide initiates TRIF-dependent signaling pathway independent of CD14. PLoS One, 2013. 8(4): p. e60078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi J, et al. , Inflammatory caspases are innate immune receptors for intracellular LPS. Nature, 2014. 514(7521): p. 187–92. [DOI] [PubMed] [Google Scholar]
- 41.Shirey KA, et al. , The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature, 2013. 497(7450): p. 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zanoni I, et al. , An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science, 2016. 352(6290): p. 1232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hashem HE, Ibrahim ZH, and Ahmed WO, Diagnostic, Prognostic, Predictive, and Monitoring Role of Neutrophil CD11b and Monocyte CD14 in Neonatal Sepsis. Dis Markers, 2021. 2021: p. 4537760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cakir Madenci O, et al. , Evaluation of soluble CD14 subtype (presepsin) in burn sepsis. Burns, 2014. 40(4): p. 664–9. [DOI] [PubMed] [Google Scholar]
- 45.Ju N, et al. , Prevention of Acute Lung Injury by a Novel CD14-Inhibitory Receptor Activator of the NF-kappaB Ligand Peptide in Mice. Immunohorizons, 2021. 5(6): p. 438–447. [DOI] [PubMed] [Google Scholar]
- 46.Keshari RS, et al. , CD14 inhibition improves survival and attenuates thrombo-inflammation and cardiopulmonary dysfunction in a baboon model of Escherichia coli sepsis. J Thromb Haemost, 2021. 19(2): p. 429–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang PH, et al. , The Dynamic Change of Immune Checkpoints and CD14+ Monocytes in Latent Tuberculosis Infection. Biomedicines, 2021. 9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fageeh HN, et al. , Gingival crevicular fluid infiltrating CD14+ monocytes promote inflammation in periodontitis. Saudi J Biol Sci, 2021. 28(5): p. 3069–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong SS, et al. , Activated CD4(+) T cells and CD14(hi)CD16(+) monocytes correlate with antibody response following influenza virus infection in humans. Cell Rep Med, 2021. 2(4): p. 100237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao SX, et al. , CD14(+) monocytes and CD163(+) macrophages correlate with the severity of liver fibrosis in patients with chronic hepatitis C. Exp Ther Med, 2020. 20(6): p. 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Messner CB, et al. , Ultra-High-Throughput Clinical Proteomics Reveals Classifiers of COVID-19 Infection. Cell Syst, 2020. 11(1): p. 11–24 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin TR, et al. , Targeting innate immunity by blocking CD14: Novel approach to control inflammation and organ dysfunction in COVID-19 illness. EBioMedicine, 2020. 57: p. 102836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fuentelsaz-Romero S, et al. , The Macrophage Reprogramming Ability of Antifolates Reveals Soluble CD14 as a Potential Biomarker for Methotrexate Response in Rheumatoid Arthritis. Front Immunol, 2021. 12: p. 776879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young JL, et al. , CD14 deficiency impacts glucose homeostasis in mice through altered adrenal tone. PLoS One, 2012. 7(1): p. e29688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernandez-Real JM, et al. , CD14 modulates inflammation-driven insulin resistance. Diabetes, 2011. 60(8): p. 2179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fang H, et al. , SIRT1 induces the accumulation of TAMs at colorectal cancer tumor sites via the CXCR4/CXCL12 axis. Cell Immunol, 2021. 371: p. 104458. [DOI] [PubMed] [Google Scholar]
- 57.Kessoku T, et al. , Endotoxins and Non-Alcoholic Fatty Liver Disease. Front Endocrinol (Lausanne), 2021. 12: p. 770986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang TT, et al. , Role of transcription factor NFAT in glucose and insulin homeostasis. Mol Cell Biol, 2006. 26(20): p. 7372–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Jesus JCR, et al. , Activation of the Adipose Tissue NLRP3 Inflammasome Pathway in Cancer Cachexia. Front Immunol, 2021. 12: p. 729182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo P, et al. , Ca(2+)-Calcineurin-NFAT pathway mediates the effect of thymol on oxidative metabolism and fiber-type switch in skeletal muscle. Food Funct, 2019. 10(8): p. 5166–5173. [DOI] [PubMed] [Google Scholar]
- 61.Reiner AP, et al. , Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arterioscler Thromb Vasc Biol, 2013. 33(1): p. 158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shen Y, et al. , STAT3-YAP/TAZ signaling in endothelial cells promotes tumor angiogenesis. Sci Signal, 2021. 14(712): p. eabj8393. [DOI] [PubMed] [Google Scholar]
- 63.Costa GC, et al. , CD14 genotype and functional dichotomy of CD14+ and CD14- cells are associated with activated immune response and development of Chagas dilated cardiomyopathy. Mem Inst Oswaldo Cruz, 2020. 115: p. e200110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stanislawski MA, et al. , Soluble CD14 Levels in the Jackson Heart Study: Associations With Cardiovascular Disease Risk and Genetic Variants. Arterioscler Thromb Vasc Biol, 2021. 41(6): p. e369–e378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Estruch M, et al. , CD14 and TLR4 mediate cytokine release promoted by electronegative LDL in monocytes. Atherosclerosis, 2013. 229(2): p. 356–62. [DOI] [PubMed] [Google Scholar]
- 66.Attia ZR, et al. , Association of CD14 genetic variants and circulating level with systemic lupus erythematosus risk in Egyptian children and adolescents. Biomark Med, 2021. 15(17): p. 1669–1680. [DOI] [PubMed] [Google Scholar]
- 67.Ferreira S, et al. , Effect of gluten-free diet on levels of soluble CD14 and lipopolysaccharide-binding protein in adult patients with celiac disease. Cent Eur J Immunol, 2021. 46(2): p. 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McGill RB, et al. , Monocyte CD14 and HLA-DR expression increases with disease duration and severity in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener, 2021: p. 1–8. [DOI] [PubMed] [Google Scholar]
- 69.Rudnik M, et al. , Elevated Fibronectin Levels in Profibrotic CD14(+) Monocytes and CD14(+) Macrophages in Systemic Sclerosis. Front Immunol, 2021. 12: p. 642891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakamizo S, et al. , Single-cell analysis of human skin identifies CD14+ type 3 dendritic cells co-producing IL1B and IL23A in psoriasis. J Exp Med, 2021. 218(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cha YJ, et al. , High Nuclear Expression of Yes-Associated Protein 1 Correlates With Metastasis in Patients With Breast Cancer. Front Oncol, 2021. 11: p. 609743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Minkov G, et al. , Prognostic value of peripheral blood CD14+HLA-DR+ monocytes in patients with acute pancreatitis. J Immunoassay Immunochem, 2021. 42(5): p. 478–492. [DOI] [PubMed] [Google Scholar]
- 73.Matsumoto K, et al. , Longitudinal immune cell monitoring identified CD14(++) CD16(+) intermediate monocyte as a marker of relapse in patients with ANCA-associated vasculitis. Arthritis Res Ther, 2020. 22(1): p. 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Umemura T, et al. , Association between serum soluble CD14 and IL-8 levels and clinical outcome in primary biliary cholangitis. Liver Int, 2017. 37(6): p. 897–905. [DOI] [PubMed] [Google Scholar]
- 75.Wang J, et al. , Association between CD14 gene polymorphisms and cancer risk: a meta-analysis. PLoS One, 2014. 9(6): p. e100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gustafson MP, et al. , Intratumoral CD14+ Cells and Circulating CD14+HLA-DRlo/neg Monocytes Correlate with Decreased Survival in Patients with Clear Cell Renal Cell Carcinoma. Clin Cancer Res, 2015. 21(18): p. 4224–33. [DOI] [PubMed] [Google Scholar]
- 77.Li K, et al. , CD14 regulates gastric cancer cell epithelialmesenchymal transition and invasion in vitro. Oncol Rep, 2013. 30(6): p. 2725–32. [DOI] [PubMed] [Google Scholar]
- 78.Prat M, et al. , Circulating CD14(high) CD16(low) intermediate blood monocytes as a biomarker of ascites immune status and ovarian cancer progression. J Immunother Cancer, 2020. 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo J, et al. , Identification of CD14 as a potential biomarker of hepatocellular carcinoma using iTRAQ quantitative proteomics. Oncotarget, 2017. 8(37): p. 62011–62028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheah MT, et al. , CD14-expressing cancer cells establish the inflammatory and proliferative tumor microenvironment in bladder cancer. Proc Natl Acad Sci U S A, 2015. 112(15): p. 4725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang L, et al. , [Effect of CD14 promoter variants on the susceptibility to laryngeal cancer]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi, 2016. 51(3): p. 197–202. [DOI] [PubMed] [Google Scholar]
- 82.Lobba AR, et al. , Differential expression of CD90 and CD14 stem cell markers in malignant breast cancer cell lines. Cytometry A, 2012. 81(12): p. 1084–91. [DOI] [PubMed] [Google Scholar]
- 83.Prakash H, et al. , CD14/TLR4 priming potentially recalibrates and exerts anti-tumor efficacy in tumor associated macrophages in a mouse model of pancreatic carcinoma. Sci Rep, 2016. 6: p. 31490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schmidt FI and Latz E, CD14-New Tricks of an Old Acquaintance. Immunity, 2017. 47(4): p. 606–608. [DOI] [PubMed] [Google Scholar]
- 85.Koniaris LG, et al. , Liver regeneration. J Am Coll Surg, 2003. 197(4): p. 634–59. [DOI] [PubMed] [Google Scholar]
- 86.McKillop IH, et al. , Molecular pathogenesis of hepatocellular carcinoma. J Surg Res, 2006. 136(1): p. 125–35. [DOI] [PubMed] [Google Scholar]
- 87.Tapping RI and Tobias PS, Soluble CD14-mediated cellular responses to lipopolysaccharide. Chem Immunol, 2000. 74: p. 108–21. [DOI] [PubMed] [Google Scholar]
- 88.Baumann CL, et al. , CD14 is a coreceptor of Toll-like receptors 7 and 9. J Exp Med, 2010. 207(12): p. 2689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.He Z, et al. , CD14 Is a Co-Receptor for TLR4 in the S100A9-Induced Pro-Inflammatory Response in Monocytes. PLoS One, 2016. 11(5): p. e0156377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gupta D, et al. , CD14 is a cell-activating receptor for bacterial peptidoglycan. J Biol Chem, 1996. 271(38): p. 23310–6. [DOI] [PubMed] [Google Scholar]
- 91.Fernandez-Ruiz I, et al. , Mitochondrial DAMPs induce endotoxin tolerance in human monocytes: an observation in patients with myocardial infarction. PLoS One, 2014. 9(5): p. e95073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kol A, et al. , Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol, 2000. 164(1): p. 13–7. [DOI] [PubMed] [Google Scholar]
- 93.Ranoa DRE, Kelley SL, and Tapping RI, Human lipopolysaccharide-binding protein (LBP) and CD14 independently deliver triacylated lipoproteins to Toll-like receptor 1 (TLR1) and TLR2 and enhance formation of the ternary signaling complex. J Biol Chem, 2013. 288(14): p. 9729–9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zheng M, Ambesi A, and McKeown-Longo PJ, Role of TLR4 Receptor Complex in the Regulation of the Innate Immune Response by Fibronectin. Cells, 2020. 9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee HK, et al. , Double-stranded RNA-mediated TLR3 activation is enhanced by CD14. Immunity, 2006. 24(2): p. 153–63. [DOI] [PubMed] [Google Scholar]
- 96.Fujikura M, et al. , CD14 and Toll-Like Receptor 4 Promote Fibrillar Abeta42 Uptake by Microglia Through A Clathrin-Mediated Pathway. J Alzheimers Dis, 2019. 68(1): p. 323–337. [DOI] [PubMed] [Google Scholar]
- 97.Lederer JA, et al. , Comparison of longitudinal leukocyte gene expression after burn injury or trauma-hemorrhage in mice. Physiol Genomics, 2008. 32(3): p. 299–310. [DOI] [PubMed] [Google Scholar]
- 98.Cornils H, et al. , Ablation of the kinase NDR1 predisposes mice to the development of T cell lymphoma. Sci Signal, 2010. 3(126): p. ra47. [DOI] [PubMed] [Google Scholar]
- 99.Patil J, et al. , Bronchoalveolar lavage cell gene expression in acute lung rejection: development of a diagnostic classifier. Transplantation, 2008. 85(2): p. 224–31. [DOI] [PubMed] [Google Scholar]
- 100.Raghavachari N, et al. , Characterization of whole blood gene expression profiles as a sequel to globin mRNA reduction in patients with sickle cell disease. PLoS One, 2009. 4(8): p. e6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berthier CC, et al. , Cross-species transcriptional network analysis defines shared inflammatory responses in murine and human lupus nephritis. J Immunol, 2012. 189(2): p. 988–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bontha SV, et al. , Effects of DNA Methylation on Progression to Interstitial Fibrosis and Tubular Atrophy in Renal Allograft Biopsies: A Multi-Omics Approach. Am J Transplant, 2017. 17(12): p. 3060–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lui JC, et al. , Spatial and temporal regulation of gene expression in the mammalian growth plate. Bone, 2010. 46(5): p. 1380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jones J, et al. , Gene signatures of progression and metastasis in renal cell cancer. Clin Cancer Res, 2005. 11(16): p. 5730–9. [DOI] [PubMed] [Google Scholar]
- 105.Stevens SL, et al. , Multiple preconditioning paradigms converge on interferon regulatory factor-dependent signaling to promote tolerance to ischemic brain injury. J Neurosci, 2011. 31(23): p. 8456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Michaelevski I, et al. , Signaling to transcription networks in the neuronal retrograde injury response. Sci Signal, 2010. 3(130): p. ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Borovecki F, et al. , Genome-wide expression profiling of human blood reveals biomarkers for Huntington’s disease. Proc Natl Acad Sci U S A, 2005. 102(31): p. 11023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grzmil M, et al. , MAP kinase-interacting kinase 1 regulates SMAD2-dependent TGF-beta signaling pathway in human glioblastoma. Cancer Res, 2011. 71(6): p. 2392–402. [DOI] [PubMed] [Google Scholar]
- 109.van Lieshout MH, et al. , NLRP3 and ASC differentially affect the lung transcriptome during pneumococcal pneumonia. Am J Respir Cell Mol Biol, 2014. 50(4): p. 699–712. [DOI] [PubMed] [Google Scholar]
- 110.Koth LL, et al. , Sarcoidosis blood transcriptome reflects lung inflammation and overlaps with tuberculosis. Am J Respir Crit Care Med, 2011. 184(10): p. 1153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Smith PJ, et al. , Mucosal transcriptomics implicates under expression of BRINP3 in the pathogenesis of ulcerative colitis. Inflamm Bowel Dis, 2014. 20(10): p. 1802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Swan C, et al. , Identifying and testing candidate genetic polymorphisms in the irritable bowel syndrome (IBS): association with TNFSF15 and TNFalpha. Gut, 2013. 62(7): p. 985–94. [DOI] [PubMed] [Google Scholar]
- 113.Holbrook JD, et al. , Deep sequencing of gastric carcinoma reveals somatic mutations relevant to personalized medicine. J Transl Med, 2011. 9: p. 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Haslett JN, et al. , Gene expression comparison of biopsies from Duchenne muscular dystrophy (DMD) and normal skeletal muscle. Proc Natl Acad Sci U S A, 2002. 99(23): p. 15000–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bachinski LL, et al. , Altered MEF2 isoforms in myotonic dystrophy and other neuromuscular disorders. Muscle Nerve, 2010. 42(6): p. 856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bialek P, et al. , Distinct protein degradation profiles are induced by different disuse models of skeletal muscle atrophy. Physiol Genomics, 2011. 43(19): p. 1075–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goh YP, et al. , Eosinophils secrete IL-4 to facilitate liver regeneration. Proc Natl Acad Sci U S A, 2013. 110(24): p. 9914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liao YL, et al. , Identification of SOX4 target genes using phylogenetic footprinting-based prediction from expression microarrays suggests that overexpression of SOX4 potentiates metastasis in hepatocellular carcinoma. Oncogene, 2008. 27(42): p. 5578–89. [DOI] [PubMed] [Google Scholar]
- 119.Niu C, et al. , The Smc5/6 Complex Restricts HBV when Localized to ND10 without Inducing an Innate Immune Response and Is Counteracted by the HBV X Protein Shortly after Infection. PLoS One, 2017. 12(1): p. e0169648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yuan S, et al. , The Prediction of Clinical Outcome in Hepatocellular Carcinoma Based on a Six-Gene Metastasis Signature. Clin Cancer Res, 2017. 23(1): p. 289–297. [DOI] [PubMed] [Google Scholar]
- 121.Edmonds RD, et al. , Transcriptomic response of murine liver to severe injury and hemorrhagic shock: a dual-platform microarray analysis. Physiol Genomics, 2011. 43(20): p. 1170–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schwientek P, et al. , Global gene expression analysis in nonfailing and failing myocardium pre- and postpulsatile and nonpulsatile ventricular assist device support. Physiol Genomics, 2010. 42(3): p. 397–405. [DOI] [PubMed] [Google Scholar]
- 123.Abcouwer SF, et al. , Effects of ischemic preconditioning and bevacizumab on apoptosis and vascular permeability following retinal ischemia-reperfusion injury. Invest Ophthalmol Vis Sci, 2010. 51(11): p. 5920–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table1: Full data table showing the pathologies, disease scores and studies associated with CD14 expressaion, generated using the base space correlation engine / disease atlas / Illumina.