Figure 2.
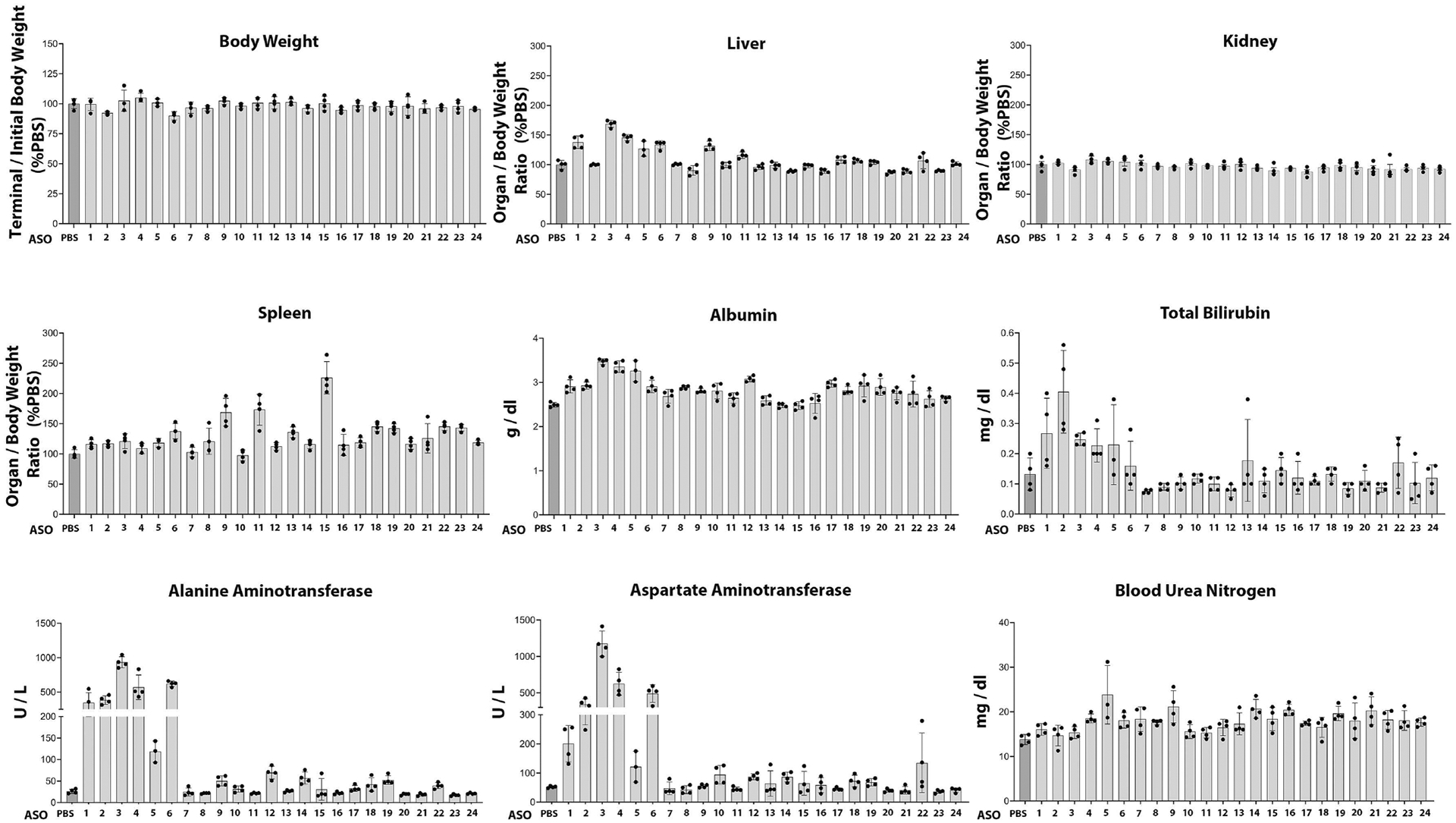
The safety profile of 24 mutant Notch3 ASOs was evaluated in mice following subcutaneous administration once a week for 4.5 weeks at 50 mg/Kg. At the end of the study, body, liver, kidney, and spleen weights were assessed, and plasma levels of alanine aminotransferase, aspartate aminotransferase, total bilirubin, albumin, and blood urea nitrogen were measured. Individual values are shown, and bars and ranges represent means ± SD; n = 4 mice for each ASO tested. Twelve ASOs considered safe were selected for subsequent activity studies.
