Scheme 1.
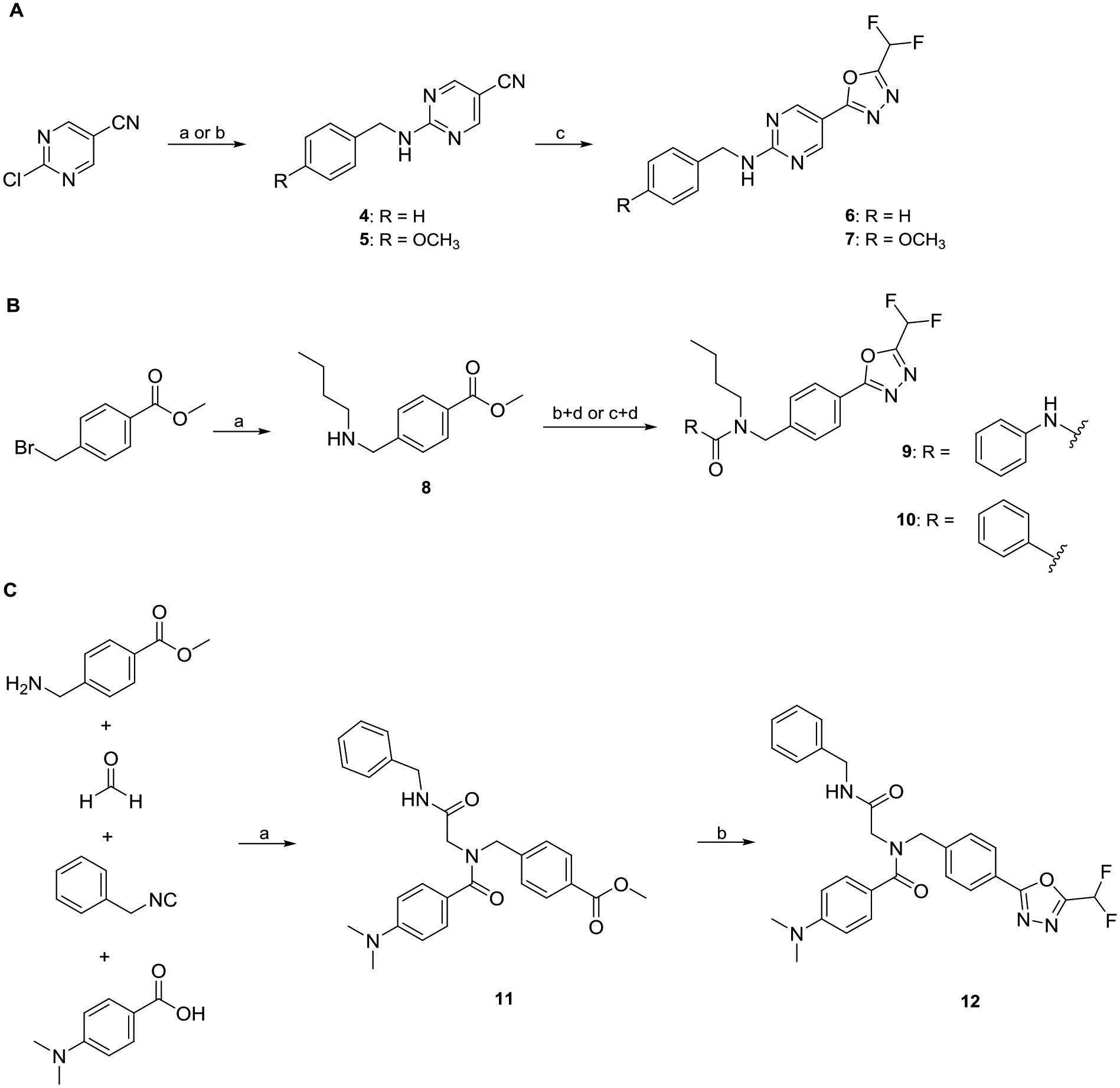
A: Synthesis of full sized HDAC6i 6 and 7. a) Benzylamine, DIPEA, EtOH, 90 °C, 18 h (4); b) 4-methoxybenzylamine, DIPEA, EtOH, 90 °C, 18 h (5); c) i: NaN3, NH4Cl, LiCl·H2O, DMF, 100 °C, 18 h; ii: DFAA, 70 °C, 18 h. B: Synthesis of nexturastat analogs 9 and 10. a) n-Butylamine, THF, rt., 3 h; b) phenyl phenylcarbamate, TEA, THF, 66 °C, 2 h (9); c) benzoyl chloride, CH2Cl2, rt., 2 h (10); d) i: hydrazine monohydrate, MeOH, 70 °C, 3 h; ii: DFAA, DMF, 70 °C, 1 h; iii: Burgess reagent, THF, 60 °C, 18 h. C: Synthesis of the peptoid-based HDACi 12. a) TEA, MeOH, rt., 72 h; b) i: hydrazine monohydrate, MeOH, 70 °C, 3 h; ii: DFAA, TEA, DMF, 70 °C, 1 h; iii: Burgess reagent, TEA, THF, 60 °C, 18 h.
