Figure 2.
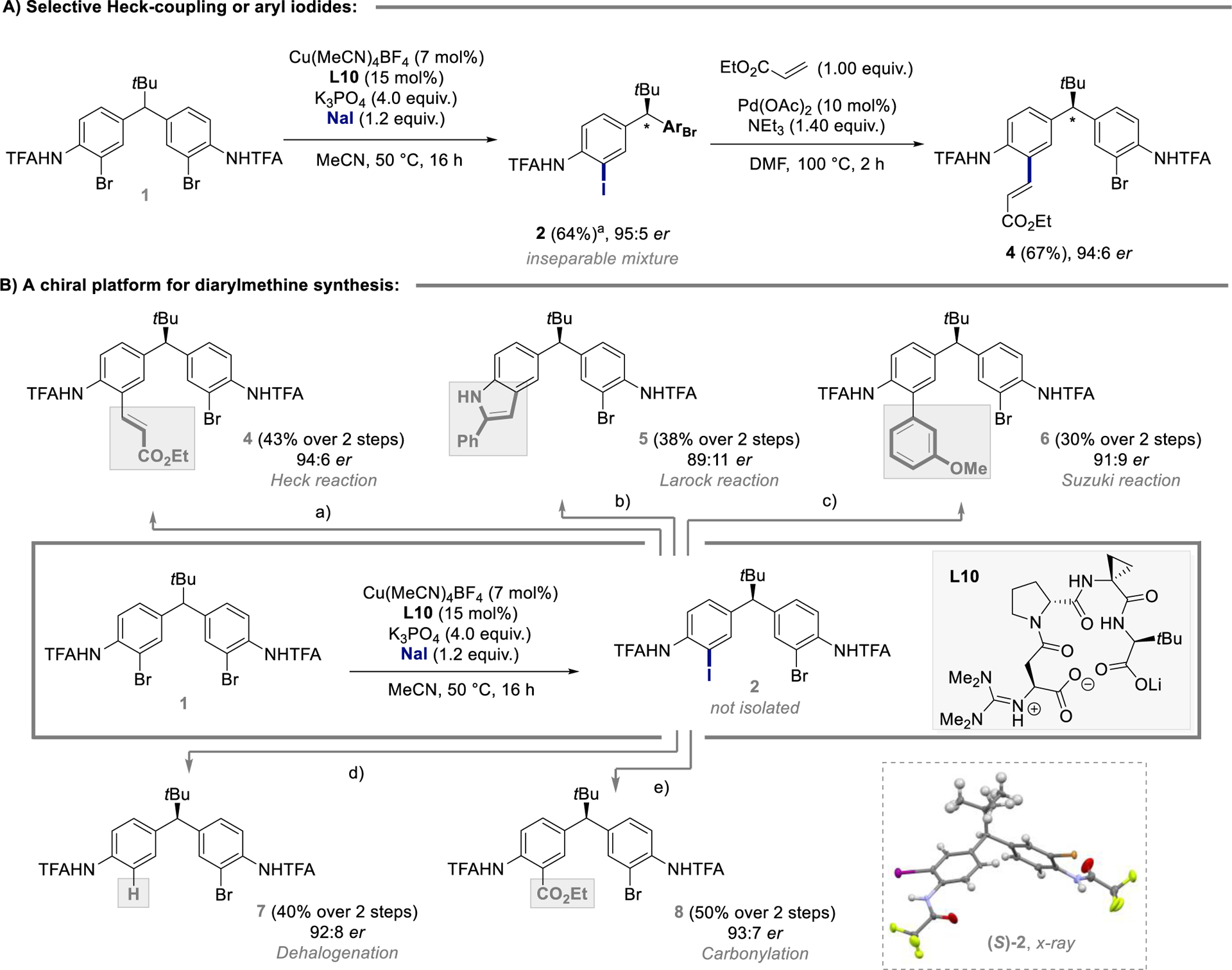
A) Identification of an asymmetric aromatic Finkelstein / Heck reaction sequence. aYield determined by 1H NMR using dibromomethane as internal standard. B) Asymmetric aromatic Finkelstein reaction as platform for the synthesis of enantioenriched diarylmethanes. Reaction conditions (0.2 mmol scale): a) Pd(OAc)2, NEt3, ethyl acrylate, DMF; b) CuI, PPh3, K3PO4, phenylacetylene, 1,4-dioxane; c) Pd(OAc)2, NEt3, 3-methoxyphenylboronic acid, toluene; d) Pd(OAc)2, NaBH4, TMEDA, DMF; e) Pd(OAc)2, NEt3, EtOH/DMF (1:4), CO. X-ray structure of 2 is shown with atomic thermal parameters calculated at 50% probability levels.
