Figure 2. LP-184 inhibits growth of pancreatic adenocarcinoma xenografts carrying DNA repair pathway mutations in PTGR1-dependnet manner.
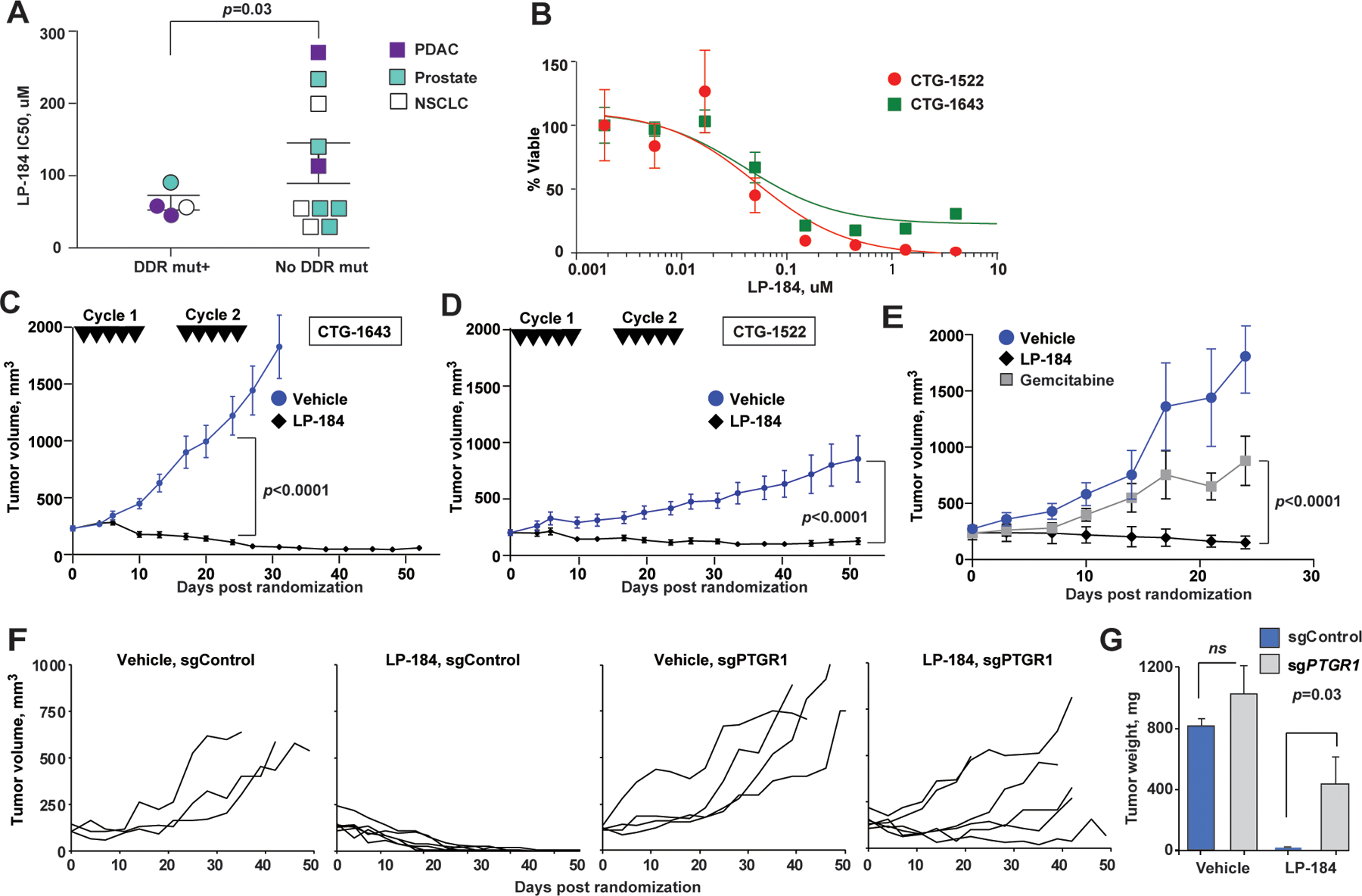
(A) Ex vivo LP-184 cytotoxicity in patient-derived xenografts correlates with presence of DDR pathway mutations. p=0.03, unpaired two-way t-test. (B) Representative LP-184 ex vivo cytotoxicity against PDX tumors of PDAC carrying pathogenic DDR pathway mutations (red circles, CTG-1522 carrying ATR I774fs; green squares, CTG-1643 carrying BRCA1 Q1460fs). (C, D) Regression of CTG-1643 (C) and CTG-1522 (D) xenografts following two cycles of LP-184 (each cycle consisted of 5 intravenous doses of LP-814 at 4 mg/kg on days 0, 2, 4, 6, 8, 16, 18, 20, 22, 24). (E) LP-184 (once weekly intraperitoneal doses of 3 mg/kg for 3 weeks) demonstrates superior efficacy compared to gemcitabine given at maximum tolerated dose (once weekly intraperitoneal dose of 50 mg/kg for 3 weeks). (F) Individual volumes of subcutaneous Capan-1 xenografts CRISPRi-depleted of PTGR1 (sgPTGR1) or sgControl-modified and treated with LP-184 at 3 mg/kg, or vehicle; p<0.0001, for tumor volume slopes of sgPTGR1 and sgControl. The schedule of intraperitoneal administration of LP-184 is shown in Figure S2B. All animals received 8–9 doses of LP-184. (G) Tumors weights at the end of experiment. p-values by unpaired two-way t-test. In C-F, linear mixed effects model was used to test differences in slopes of tumor volumes.
