Abstract
The pharmacokinetics of the antifungal pradimicin derivative BMS 181184 in plasma of normal, catheterized rabbits were characterized after single and multiple daily intravenous administrations of dosages of 10, 25, 50, or 150 mg/kg of body weight, and drug levels in tissues were assessed after multiple dosing. Concentrations of BMS 181184 were determined by a validated high-performance liquid chromatography method, and plasma data were modeled into a two-compartment open model. Across the investigated dosage range, BMS 181184 demonstrated nonlinear, dose-dependent kinetics with enhanced clearance, reciprocal shortening of elimination half-life, and an apparently expanding volume of distribution with increasing dosage. After single-dose administration, the mean peak plasma BMS 181184 concentration (Cmax) ranged from 120 μg/ml at 10 mg/kg to 648 μg/ml at 150 mg/kg; the area under the concentration-time curve from 0 to 24 h (AUC0–24) ranged from 726 to 2,130 μg · h/ml, the volume of distribution ranged from 0.397 to 0.799 liter/kg, and the terminal half-life ranged from 4.99 to 2.31 h, respectively (P < 0.005 to P < 0.001). No drug accumulation in plasma occurred after multiple daily dosing at 10, 25, or 50 mg/kg over 15 days, although mean elimination half-lives were slightly longer. Multiple daily dosing at 150 mg/kg was associated with enhanced total clearance and a significant decrease in AUC0–24 below the values obtained at 50 mg/kg (P < 0.01) and after single-dose administration of the same dosage (P < 0.05). Assessment of tissue BMS 181184 concentrations after multiple dosing over 16 days revealed substantial uptake in the lungs, liver, and spleen and, most notably, dose-dependent accumulation of the drug within the kidneys. These findings are indicative of dose- and time-dependent elimination of BMS 181184 from plasma and renal accumulation of the compound after multiple dosing.
Concurrent with the increasing number of patients with impaired host defenses, invasive fungal infections have emerged as important causes of morbidity and mortality in hospitals (1, 12). Therapeutic options have been expanded by the introduction of antifungal triazoles (15) and lipid formulations of amphotericin B (13), but each of the individual compounds has its specific limitations of safety, spectrum, and efficacy (11, 23). With the emergence of unusual fungal pathogens and drug resistance, there is an indisputable need for new, well-tolerated antifungal agents with novel modes of action and improved efficacy in immunocompromised patient populations.
The pradimicins are a new class of fermentation-derived antifungal antibiotics structurally characterized by a dihydrobenzonaphthacene quinone skeleton substituted with a d amino acid and a disaccharide side chain (16). The proposed mechanism of action of these agents consists of a calcium-dependent complexing with the saccharide portion of fungal cell wall mannoproteins, which leads to perturbation of the integrity and function of the fungal cell membrane and, ultimately, cell death (21). The pradimicins have non-cross-resistant, broad-spectrum fungicidal activity in vitro, being effective against Candida spp., Aspergillus spp., Cryptococcus neoformans, and other fungal pathogens (7, 14, 17, 18). Preclinical in vivo studies have demonstrated promising safety and efficacy in experimental models of invasive fungal infections in both normal and immunocompromised animals (9, 10, 14, 17–20).
Little is known, however, about the specific disposition of this new class of compounds in plasma and tissues. The purpose of this study was therefore to investigate the plasma pharmacokinetics and tissue distribution of BMS 181184, a water-soluble pradimicin FA-2 derivative with potent in vitro and in vivo antifungal activity.
MATERIALS AND METHODS
Study drug.
BMS 181184 (250-mg vials; Bristol-Myers Squibb, Princeton, N.J.) was provided as a lyophilized powder, maintained at 4°C, and freshly reconstituted in sterile normal saline–sterile 5% dextrose in water (1:1, vol/vol) to a 50-mg/ml solution prior to dosing of animals.
Animals.
Healthy female New Zealand White rabbits (Hazleton, Denver, Pa.) weighing 2.5 to 3.5 kg were used in all experiments. They were individually housed and maintained with water and standard rabbit feed ad libitum according to National Institutes of Health guidelines for laboratory animal care (4) and in fulfillment of American Association for Accreditation of Laboratory Animal Care criteria. Vascular access was established in each rabbit by the surgical placement of a subcutaneous silastic central venous catheter as previously described (22).
Single-dose studies.
Four groups of three rabbits each were studied. Animals received BMS 181184 at 10, 25, 50, or 150 mg/kg of body weight as a single steady intravenous (i.v.) bolus (2 mg/s). Plasma samples were drawn immediately before administration of the compound and then at 0.16, 0.5, 1, 2, 3, 5, 6, 7, and 24 h after its administration.
Multiple-dose studies.
Four groups of three rabbits each were studied. After the single-dose pharmacokinetics study was completed, the animals continued to receive BMS 181184 at 10, 25, 50, or 150 mg/kg of body weight as a single i.v. bolus once a day for a total of 16 days. On day 15, plasma samples were drawn immediately before dosing and then at 0.16, 0.5, 1, 2, 3, 5, 6, 7, and 24 h postdosing. On day 16, rabbits were euthanized by i.v. administration of pentobarbital 1 h after administration of the compound and samples from the brain, cerebrospinal fluid (CSF), vitreous fluid, lung, liver, spleen, and kidney were obtained for determination of their drug levels at the time of near-peak levels in the plasma.
For animals receiving the 50- and 150-mg/kg dosage levels, hepatic toxicity and renal toxicity were monitored by performing biochemical profiles of blood urea nitrogen (BUN), serum creatinine, bilirubin, serum aspartate aminotransferase (AST), and serum alanine aminotransferase (ALT) on the first and last days of administration of BMS 181184. All animals were clinically evaluated each day and weighed once weekly.
Processing of blood and tissue specimens.
Blood samples were collected in heparinized syringes. Plasma was separated by centrifugation and stored at −80°C until assayed. Tissue samples were carefully dissected and stored at −80°C. Before the assay, the tissue specimens were thawed, and three portions of approximately 1 g each were weighed for each sample (balance model AE 163; Mettler Instrument Corp., Hightstown, N.J.). The specimens were thoroughly rinsed with phosphate-buffered saline, pH 7.4 (Quality Biological, Inc., Gaithersburg, Md.). Buffer solution remaining on the tissue surface was removed by blotting with Micro Wipes (Scott Paper Company, Philadelphia, Pa.). The specimens were then reweighed and homogenized with phosphate-buffered saline, pH 7.4 (1:3 [wt/wt]). Tissue specimens were homogenized four times, for 30 s each, in a Tissumizer (Tekmar, Cincinnati, Ohio) equipped with a 10N head. To avoid tissue heating, homogenization was performed with the sample being placed in an ice bucket. A 100-μl sample of the resulting homogenate was subjected to assay, and the BMS 181184 concentration per gram of tissue was calculated. Standards and quality control samples were similarly prepared by homogenizing normal tissues in phosphate-buffered saline, pH 7.4 (1:3 [wt/wt]), and adding known amounts of the compound. Blank samples of these homogenates, containing no BMS 181184, were also extracted to ensure the absence of interfering peaks.
Analytical method.
Levels of BMS 181184 in plasma and tissue homogenates were determined by an accurate, sensitive, reproducible, and specific high-performance liquid chromatographic (HPLC) method developed and fully validated at the Bristol-Myers Squibb Pharmaceutical Research Institute, Princeton, N.J. The reference standard of the compound (Bristol-Myers Squibb, Princeton, N.J.) was ≥94% pure. The method involved precipitation of proteins by addition of 1.2 ml of methanol to each standard, control, or unknown rabbit plasma specimen, or tissue homogenate, followed by centrifugation and removal of the methanolic layer. The methanolic supernatant was then evaporated to dryness at 40°C under a stream of nitrogen, and the sample was reconstituted in 250 μl of the mobile phase (50 mM sterile potassium phosphate buffer–acetonitrile, 80/20 [vol/vol]; Fisher Scientific, Fair Lawn, N.J.) for injection onto the HPLC column. The injection volume was 180 μl, and the flow rate was 1.3 ml/min. BMS 181184 eluted at approximately 3.5 to 4.5 min from a C18 analytical column maintained at 35°C (Ultrasphere; Beckman Instruments, Fullerton, Calif.) and was detected by its UV absorbance at 510 nM. Quantification was based on the peak area-concentration response of the calibration standard. Ten-point standard curves were linear from 0 to 200 μg/ml, with r2 values of greater than 0.97. The lower limit of quantification was 0.2 μg/ml. Accuracies were within 15%, and intra- and interday variability (precision) was <10%.
Pharmacokinetic analysis.
Pharmacokinetic parameters for BMS 181184 were determined by performing both compartmental and noncompartmental analyses. Concentrations of BMS 181184 were fit to a two-compartment open model, with i.v. bolus input and elimination from the central compartment, by iterative weighted nonlinear least-squares regression with the ADAPT II computer program (5). Weighting was achieved by using the inverse of the square root of the observation variance. Model selection was guided by Akaike’s information criterion (24). The model fit the data well, with a mean coefficient of determination (r2) of 0.992 (r2 = 0.969 to 1.00), and the regression lines through the plot of observed versus fitted concentrations did not differ from the line of identity. Fitted parameters were total clearance (CLT), distributional clearance (CLD), volume of distribution (V), volume of distribution of the central (V1) and the peripheral (Vp) compartments, distributional half-life (t1/2α), and elimination half-life (t1/2β). Model-independent parameters of maximum (Cmax) and minimum (Cmin) concentrations were determined directly from concentration-time profiles. The area under the plasma concentration-time curve from 0 to 24 h (AUC0–24) was determined by the linear trapezoidal rule (8).
Statistical analysis.
Differences between the means of pharmacokinetic parameters across the four dosage levels were evaluated by the Kruskal-Wallis nonparametric analysis of variance (ANOVA) test. For comparison of two dosage levels or dosage schedules, Student’s t test or Welch’s t test was used, as appropriate. Simple linear regression and one-way ANOVA were utilized to assess dose linearity. A two-tailed P value of <0.05 was considered statistically significant.
RESULTS
Single-dose studies.
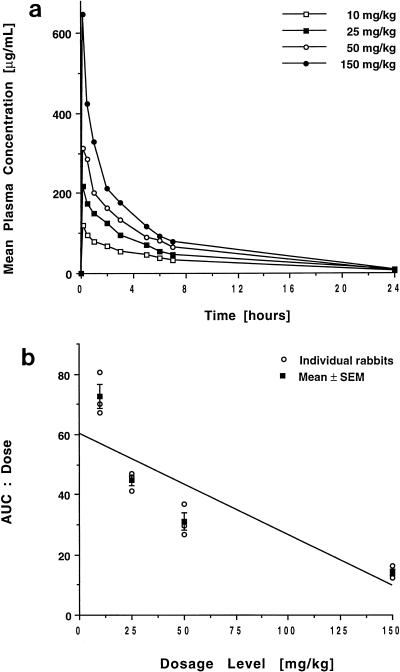
The concentration-versus-time curves of BMS 181184 in plasma following single-dose administration are shown in Fig. 1a, and calculated pharmacokinetic parameters for the four dosage levels are listed in Table 1.
FIG. 1.
Single-dose plasma pharmacokinetics of BMS 181184. (a) Concentration-versus-time curves after administration of 10, 25, 50, and 150 mg of the compound/kg. Each point plots the mean level for three rabbits each at that time. (b) Plot of the AUC0–24/dose ratios for each rabbit and the mean ± the standard error of the mean (SEM) for each dosage group. For a drug that follows linear kinetics, such a plot would have a slope of 0. For BMS 181184, however, the significantly negative slope of the means (P < 0.001; ANOVA) is indicative of the compound’s nonlinear kinetics.
TABLE 1.
Single-dose pharmacokinetics of BMS 181184 in plasmaa
| Drug dose (mg/kg) | Cmaxb (μg/ml) | Cmin (μg/ml) | AUC0–24b (μg · h/ml) | Vc (liter/kg) | V1 (liter/kg) | Vp (liter/kg) | CLTd (liter/h) | CLDe (liter/h) | t1/2α (h) | t1/2βf (h) |
|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 120 ± 7 | 5 ± 0.5 | 726 ± 40 | 0.397 ± 0.019 | 0.244 ± 0.013 | 0.153 ± 0.017 | 0.058 ± 0.005 | 0.361 ± 0.140 | 0.221 ± 0.062 | 4.99 ± 0.71 |
| 25 | 218 ± 11 | 8 ± 0.5 | 1,120 ± 44 | 0.506 ± 0.038 | 0.315 ± 0.098 | 0.191 ± 0.067 | 0.103 ± 0.009 | 0.610 ± 0.276 | 0.148 ± 0.050 | 3.50 ± 0.08 |
| 50 | 312 ± 4 | 5 ± 0.8 | 1,551 ± 146 | 0.545 ± 0.039 | 0.366 ± 0.033 | 0.179 ± 0.006 | 0.097 ± 0.009 | 0.270 ± 0.062 | 0.341 ± 0.105 | 4.08 ± 0.08 |
| 150 | 648 ± 9 | 9 ± 1.1 | 2,130 ± 167 | 0.799 ± 0.056 | 0.404 ± 0.037 | 0.395 ± 0.093 | 0.259 ± 0.023 | 0.902 ± 0.194 | 0.142 ± 0.019 | 2.31 ± 0.21 |
All values are expressed as means ± standard errors of the means.
P < 0.001 by Kruskal-Wallis nonparametric ANOVA.
P < 0.005 by Kruskal-Wallis nonparametric ANOVA; 150 versus either 10, 25, or 50 mg/kg, P < 0.05 by the Student t test.
P < 0.001 by Kruskal-Wallis nonparametric ANOVA; 25 or 50 versus 10 mg/kg, P < 0.05, and 150 versus 50 or 25 mg/kg, P < 0.01, both by the Student t test.
150 versus 50 mg/kg, P < 0.05 by the Student t test.
P < 0.001 by Kruskal-Wallis nonparametric ANOVA; 150 versus either 10, 25, or 50 mg/kg, P < 0.05 by the Student t test.
Administration of the compound at dosages from 10 to 150 mg/kg resulted in escalating peak plasma BMS 181184 levels ranging from 120 ± 7 to 648 ± 9 μg/ml (mean ± standard error of the mean). The drug was rapidly distributed, while its elimination occurred more slowly, with a terminal half-life ranging from 4.99 to 2.31 h. Minimum concentrations in plasma at 24 h postdosing averaged 6.75 μg/ml and did not change significantly with the dose. Over the observed dose range, increases in dosage resulted in subproportional but significant (P < 0.001) increases in both Cmax and AUC0–24; this coincided with an increase in CLT and a reduced t1/2β at the 150-mg/kg dosage level (P < 0.001 for both), consistent with a nonlinear disposition of the compound (Fig. 1b). V was approximately equivalent to that of total body water but increased in a dose-dependent manner (P < 0.005). Interindividual variability was low in all dosing groups, with coefficients of variation for the AUC0–24 of 9.6, 6.85, 16.35, and 13.59 for the 10-, 25-, 50-, and 150-mg/kg dosage levels, respectively.
Multiple-dose studies.
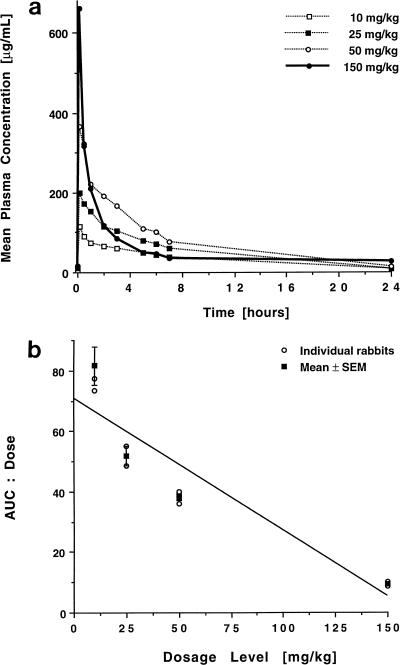
The concentration-versus-time profiles of BMS 181184 in plasma following once-daily administration of 10, 25, 50, or 150 mg/kg over 15 days are shown in Fig. 2a, and the corresponding pharmacokinetic parameters are listed in Table 2.
FIG. 2.
Multiple-dose plasma pharmacokinetics of BMS 181184. (a) Concentration-versus-time curves after administration of 10, 25, 50, and 150 mg of the compound/kg over 15 days. Each point plots the mean level for three rabbits each at that time. (b) Plot of the ratios of AUC0–24/dose for each rabbit and the mean ± the standard error of the mean (SEM) for each dosage group. Similar to the single-dose data, the significantly negative slope of the means (P < 0.005; ANOVA) demonstrates the nonlinear kinetics of BMS 181184 after multiple dosing.
TABLE 2.
Pharmacokinetics of BMS 181184 in plasma after multiple dosing over 15 daysa
| Drug dose (mg/kg · day) | Cmaxb (μg/ml) | Cmin (μg/ml) | AUC0–24c (μg · h/ml) | Vd (liter/kg) | V1 (liter/kg) | Vpe (liter/kg) | CLTf (liter/h) | CLDg (liter/h) | t1/2αh (h) | t1/2βi (h) |
|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 116 ± 6 | 9 ± 4.0 | 816 ± 63 | 0.508 ± 0.076 | 0.273 ± 0.019 | 0.235 ± 0.079 | 0.049 ± 0.003 | 0.361 ± 0.099 | 0.229 ± 0.020 | 7.33 ± 1.26 |
| 25 | 198 ± 8 | 8 ± 1.5 | 1,298 ± 83 | 0.672 ± 0.129 | 0.472 ± 0.054 | 0.200 ± 0.076 | 0.082 ± 0.009 | 0.143 ± 0.019 | 0.617 ± 0.100 | 6.00 ± 0.62 |
| 50 | 366 ± 24 | 15 ± 3.2 | 1,911 ± 55 | 0.497 ± 0.071 | 0.312 ± 0.023 | 0.185 ± 0.093 | 0.080 ± 0.010 | 0.351 ± 0.274 | 0.563 ± 0.282 | 5.00 ± 1.38 |
| 150 | 662 ± 23 | 26 ± 4.4 | 1,385 ± 81 | 1.013 ± 0.133 | 0.348 ± 0.034 | 0.665 ± 0.109 | 0.407 ± 0.028 | 0.840 ± 0.123 | 0.162 ± 0.041 | 2.21 ± 0.51 |
All values are expressed as means ± standard errors of the means. Data of the 25-mg/kg dosage level stem from two rabbits only; in the third animal, the catheter was clogged.
P < 0.001 by Kruskal-Wallis nonparametric ANOVA.
P < 0.005 by Kruskal-Wallis nonparametric ANOVA; 150 versus 50 mg/kg, P < 0.01 by the Student t test.
150 versus 10 or 50 mg/kg, P < 0.05 by the Student t test.
150 versus 10 or 50 mg/kg, P < 0.05 by the Student t test.
P < 0.005 by Kruskal-Wallis nonparametric ANOVA; 25 or 50 versus 10 mg/kg, P < 0.01, and 150 versus either 50, 25, or 10 mg/kg, P < 0.01, both by the Student t test.
150 versus 25 or 10 mg/kg, P < 0.05 by the Student t test.
P < 0.05 by Kruskal-Wallis nonparametric ANOVA; 25 or 50 versus either 10 or 150 mg/kg, P < 0.05 by the Student t test.
150 versus 10 or 25 mg/kg, P < 0.05 by the Student t test.
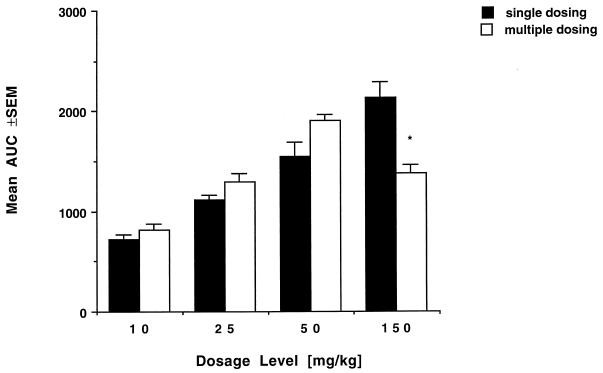
Peak plasma BMS 181184 levels after multiple dosing were not different from those observed after administration of a single dose. Cmin values increased with increasing dosage, but this trend was not statistically significant. Cmin values at the 50- and 150-mg/kg dosages, however, were significantly greater than those measured after single-dose administration (15 ± 3.2 versus 5 ± 0.8 μg/ml and 26 ± 4.4 versus 9 ± 1.1 μg/ml, respectively; P < 0.05). Drug disposition after multiple dosing retained its nonlinear character, as evident from Fig. 2b. There was an unexpected, significant decrease in the AUC0–24 at the 150-mg/kg dosage level compared to the value obtained after single-dose administration (P < 0.05). Moreover, the AUC0–24 at the 150-mg/kg dosage level was significantly lower than the AUC0–24 obtained at the 50-mg/kg dosage level (P < 0.01) (Fig. 3). This again coincided with an increase in CLT (P < 0.005) over the observed dosage range. In addition, at the highest dosage level, CLT was significantly greater after multiple dosing than after single-dose administration (P < 0.05). There were no significant differences in V for multiple and single dosing except for an apparently further-increased volume of distribution at the 150-mg/kg dose level after repeat administration of the drug. Interindividual variability was low in all groups, with coefficients of variation for AUC0–24 of 13.37, 9.04, 5.02, and 10.18 for the 10-, 25-, 50-, and 150-mg/kg · day dosage levels, respectively.
FIG. 3.
AUC0–24 values at the four investigated dosage levels after single-dose and multiple-dose administration of BMS 181184 (mean values for three rabbits each ± standard errors of the means [SEM]). Note the decrease in the AUC0–24 after multiple once-daily dosing at 150 mg/kg compared to the next-lower dosage level (∗, P < 0.01; Student’s t test) and compared to the AUC0–24 obtained after one single dose of 150 mg/kg (∗, P < 0.05; Student’s t test).
Distribution in tissues.
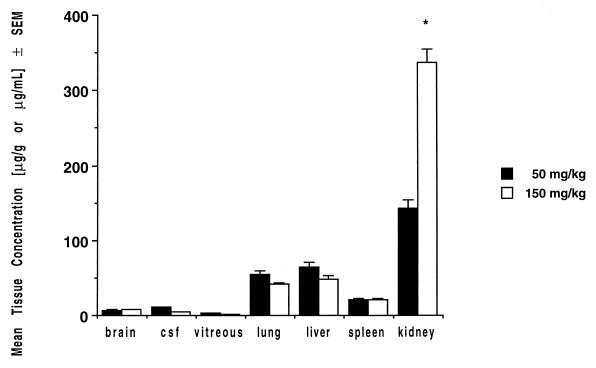
Mean levels of BMS 181184 in tissues at near-peak plasma concentrations after multiple dosing over 16 days are shown in Table 3. The highest drug levels were detected in the kidney, where the compound accumulated progressively with increasing dosages up to levels exceeding concurrent plasma concentrations by a factor of 1.6; substantial, apparently AUC-proportional drug disposition occurred in the lung, liver, and spleen, with concentrations well above the MICs for most pathogenic fungi (Fig. 4). Drug levels in brain tissue, CSF, and vitreous fluid were lower than those measured in other tissues but were detectable at concentrations exceeding 1 μg/g or 1 μg/ml.
TABLE 3.
Levels of BMS 181184 in tissues after multiple dosing over 16 days
| Drug dose (mg/kg · day) | BMS 181184 level (mean ± SEM) ina:
|
||||||
|---|---|---|---|---|---|---|---|
| Brain (μg/g) | CSF (μg/ml) | Vitreous fluid (μg/ml) | Lung (μg/g) | Liver (μg/g) | Spleen (μg/g) | Kidneyb (μg/g) | |
| 10 | 4.30 ± 0.72 [0.0052] | NDc | 2.84 ± 0.28 [0.0034] | 28.18 ± 0.90 [0.0345] | 30.37 ± 5.10 [0.0372] | 11.48 ± 0.55 [0.0140] | 37.45 ± 5.75 [0.0458] |
| 25 | 4.76 ± 0.81 [0.0036] | ND | 2.93 ± 0.35 [0.0022] | 38.09 ± 2.77 [0.0293] | 54.33 ± 2.19 [0.0418] | 17.52 ± 1.90 [0.0134] | 72.66 ± 3.39 [0.0559] |
| 50 | 6.55 ± 0.72 [0.0034] | 10.58 ± 1.00 [0.0056] | 3.67 ± 0.19 [0.0019] | 53.86 ± 5.19 [0.0287] | 64.02 ± 6.05 [0.0335] | 20.73 ± 1.02 [0.0108] | 142.4 ± 12.60 [0.0745] |
| 150 | 7.65 ± 0.89 [0.0055] | 4.06 ± 0.83 [0.0029] | 1.75 ± 0.24 [0.0012] | 41.31 ± 2.77 [0.0298] | 48.01 ± 4.38 [0.0346] | 20.93 ± 1.05 [0.0151] | 336.59 ± 17.98 [0.2430] |
Values in brackets are ratios of mean tissue levels to mean plasma AUC0–24s after multiple dosing.
P < 0.001 by Kruskal-Wallis nonparametric ANOVA.
ND, not determined.
FIG. 4.
Concentrations of BMS 181184 in tissues after multiple once-daily dosing over 16 days (mean values for three rabbits each ± standard errors of the means [SEM]; 50- and 150-mg/kg dosage levels only). Note the apparent accumulation of drug within the kidney (∗, P < 0.001 versus 50 mg/kg; Student’s t test).
Toxicity.
There were no statistically significant differences in the mean plasma creatinine, BUN, bilirubin, and hepatic transaminase levels when values determined after 15 days of treatment were compared to those obtained at baseline. One rabbit at the 150-mg/kg dosage level had elevated plasma creatinine and BUN values at the end of treatment (1.5 and 33 mg/dl, respectively). Throughout administration of the compound, no apparent clinical abnormalities were observed in BMS 181184-treated rabbits and no abnormal weight changes were noted. However, the body fluids and skin of BMS 181184-treated rabbits showed a red discoloration, and, similarly, most of their tissues exhibited red discoloration at autopsy.
DISCUSSION
BMS 181184 was the first member of the pradimicin family of antifungal antibiotics to be selected for clinical development. It exemplifies the potent and broad-spectrum activity of this novel class of antifungal compounds against clinically relevant fungi both in vitro and in vivo. Considering also their unique fungicidal mechanism of action and their lack of cross-resistance with amphotericin B and antifungal triazoles, the pradimicins, as a class, clearly warrant further investigation for their use in treatment of invasive fungal infections. The present study was performed in order to characterize the compartmental pharmacokinetics of BMS 181184 and to quantify the concentrations of the compound achievable at clinically relevant peripheral sites.
Independent of the duration of administration, BMS 181184 demonstrated nonlinear, dose-dependent kinetics with significant differences in dose-normalized Cmax and AUC0–24 values across the dosage range assessed in this study. Plasma data fit well to a two-compartment open model, with a rapid initial distribution followed by a slower elimination from the central compartment. Consistent with a nonlinear disposition, there was an increase in clearance and a reciprocal shortening of the drug’s t1/2β with increasing dosage. The V was approximately equivalent to that of total body water but increased in a nonlinear fashion at the highest dosage level.
Multiple once-daily dosing over 15 days did not result in drug accumulation in the plasma. Indeed, there was a significant decrease in the AUC0–24 at the 150-mg/kg dosage level compared to the corresponding value after administration of one single dose and the AUC obtained after multiple dosing at 50 mg/kg. This coincided with a significant increase in CLT and a decrease in the t1/2β but also an expansion of the V.
Bearing in mind the limitations of tissue concentration ratios taken from a single time point of the dosing interval (6), there was substantial uptake of the drug in the lung, liver, and spleen after multiple dosing. Most notably, however, BMS 181184 appeared to accumulate within the kidney in an almost dose-proportional manner, with concentrations six to eight times higher than simultaneously measured concentrations in liver and lung tissues, respectively, and exceeding corresponding plasma drug concentrations by a factor of 1.6 after multiple once-daily dosing at 150 mg/kg.
Although the metabolic pathways of BMS 181184 have not yet been elucidated, renal mechanisms appear to play an important role in the disposition of this pradimicin derivative. On-file data from unpublished studies by the manufacturer, using rats and BMS 181184 dosages of 12, 60, and 300 mg/kg, showed that up to 58% of a single dose could be detected in the urine within the first 24 h after administration. Along with nonlinear increases in the AUC, the CLT, renal clearance, and urinary recovery as a percentage of the dose, as well as the compound’s V, increased with the dose. Values for renal clearance were lower than the estimated value for the glomerular filtration rate in the rat. After multiple once-daily dosing at 300 mg/kg, urinary recovery was greater on day 24 than on day 1, whereas there were no differences at the 10- and 60-mg/kg dose levels (2).
Our findings are well in agreement with those obtained in the rat studies. Taken together, they suggest saturable renal tubular reabsorption of the compound, resulting in enhanced renal excretion and/or accumulation within the kidney with increasing dosage and time. However, the observed two-step increase in CLT (i.e., an initial change from 10 to 25 and 50 mg/kg and a second major change at the highest dosage level) makes it also likely that BMS 181184 undergoes significant protein binding and that protein binding becomes saturated at higher concentrations and/or prolonged exposure, leading to renal excretion of free drug via glomerular filtration and accumulation in the kidney.
Good penetration into CSF and/or brain tissue is essential for any drug targeted for the treatment of invasive fungal infections. Although the levels of penetration of BMS 181184 into CSF and brain tissue were relatively low, the measured concentrations reflect only a static assessment of a dynamic process, with the possibility of a different equilibrium at later time points and completely different states of tissue inflammation and/or necrosis.
Since the assessment of toxicity was not the primary goal of our study, no tissue from the liver or kidney was sampled for histological examination. Nevertheless, with the exception of a variable red discoloration of body fluids and tissues, BMS 181184 appeared to be well tolerated over a typical treatment duration, with no evidence of clinical or laboratory abnormalities or abnormal weight changes. However, the first phase I multiple-dose study in normal human volunteers revealed increases in hepatic transaminase levels which resulted in the discontinuation of clinical development of the compound. In that study, transient, apparently dose-related increases in ALT (less than or equal to World Health Organization [WHO] grade 2) and, to a lesser extent, AST and gamma-glutamyltranspeptidase were observed. The two dose levels studied were 0.75 and 1.5 mg/kg, each given every 12 h over 7 days. Overall, 9 of 12 subjects developed abnormal ALT values. At the 0.75-mg/kg dose level, four of six subjects exhibited a WHO grade I ALT value (1.25 to 2.5 times the upper normal limit); at the 1.5-mg/kg dose level, five of six subjects had an elevated ALT level, two of them being in the WHO grade 2 range (2.6 to 5 times the upper normal limit) (3). In preclinical toxicity studies performed by the manufacturer, monkeys, mice, and rats did not display evidence of hepatic toxicity. Only when dogs received multiple doses of greater than 60 times (i.e., 90 mg/kg/day) the dosage administered to human volunteers were mild elevations of ALT levels and subacute perivascular inflammation observed (2). Thus, there was no animal model which reliably predicted hepatic toxicity of BMS 181184 in humans.
In summary, BMS 181184, the first derivative of a new and unique class of antifungal antibiotics selected for further development, demonstrated nonlinear plasma pharmacokinetics, with dose- and time-dependent increases in clearance at high dosages and evidence of dose-dependent accumulation within the kidney and substantial penetration into most peripheral sites of clinical relevance. Increased elimination at dosages potentially required for therapeutic efficacy might be overcome by dividing the administration of the drug. High-level efficacy in vivo and suitable pharmacokinetic properties would favor the further development of congeners of this promising class of compounds.
REFERENCES
- 1.Beck-Sague C M, Jarvis W R the National Nosocomial Infections Surveillance System. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 2.Bristol-Myers Squibb. BMS 181184 investigator brochure, version 1. Princeton, N.J: Bristol-Myers Squibb; 1995. [Google Scholar]
- 3.Bristol-Myers Squibb. Unpublished data.
- 4.Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the care and use of laboratory animals. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- 5.D’Argenio D Z, Schumitzky A. Adapt II user’s guide. University of Southern CaliforniaLos Angeles: Biomedical Simulations Resource; 1990. [Google Scholar]
- 6.Drusano G L, Liu W, Perkins R, Madu A, Madu C, Mayers M, Miller M H. Determination of robust ocular pharmacokinetic parameters in serum and vitreous humor of albino rabbits following systemic administration of ciprofloxacin from sparse data sets by using IT2S, a population pharmacokinetic modeling program. Antimicrob Agents Chemother. 1995;39:1683–1687. doi: 10.1128/aac.39.8.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung-Tomc J C, Minassian B, Huczko E, Kolek B, Bonner D P, Kessler R E. In vitro antifungal and fungicidal spectra of a new pradimicin derivative, BMS-181184. Antimicrob Agents Chemother. 1995;39:295–300. doi: 10.1128/aac.39.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1982. pp. 455–459. [Google Scholar]
- 9.Gonzalez C E, Shetty D, Giri N, Love W, Klygis K, Lyman C, Bacher J, Walsh T J. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Efficacy of pradimicin against disseminated candidiasis, abstr. F181; p. 131. [Google Scholar]
- 10.Gonzalez C E, Shetty D, Giri N, Love W, Klygis K, Sein T, Lyman C, Bacher J, Walsh T J. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Efficacy of pradimicin against invasive pulmonary aspergillosis, abstr. F182; p. 131. [Google Scholar]
- 11.Graybill, J. R. 1996. The future of antifungal therapy. Clin. Infect. Dis. 22(Suppl. 2):S166–S178. [DOI] [PubMed]
- 12.Groll A H, Shah P M, Mentzel C, Schneider M, Just-Nuebling G, Huebner K. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J Infect. 1996;33:23–32. doi: 10.1016/s0163-4453(96)92700-0. [DOI] [PubMed] [Google Scholar]
- 13.Hiemenz, J. W., and T. J. Walsh. 1996. Lipid formulations of amphotericin B: recent progress and future directions. Clin. Infect. Dis. 22(Suppl. 2):S133–S144. [DOI] [PubMed]
- 14.Kakushima M, Masuyoshi S, Hirano M, Shinoda M, Ohta A, Kamei H, Oki T. In vitro and in vivo antifungal activities of BMY-28864, a water-soluble pradimicin derivative. Antimicrob Agents Chemother. 1991;35:2185–2190. doi: 10.1128/aac.35.11.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kauffman, C. A. 1996. Role of azoles in antifungal therapy. Clin. Infect. Dis. 22(Suppl. 2):S148–S153. [DOI] [PubMed]
- 16.Oki T, Konishi M, Tomatsu K, Tomita K, Saitoh K, Tsunakawa M, Nishio M, Myaki T, Kawaguchi H. Pradimicin, a novel class of potent antifungal antibiotics. J Antibiot. 1988;41:1701–1704. doi: 10.7164/antibiotics.41.1701. [DOI] [PubMed] [Google Scholar]
- 17.Oki T, Tenmyo O, Hirano M, Tomatsu K, Kamei H. Pradimicins A, B and C: new antifungal antibiotics. II. In vitro and in vivo biological activities. J Antibiot. 1990;43:763–770. doi: 10.7164/antibiotics.43.763. [DOI] [PubMed] [Google Scholar]
- 18.Oki T, Kakushima M, Hirano M, Takahashi A, Ohta A, Masuyoshi S, Hatori M, Kamei H. In vitro and in vivo antifungal activities of BMS-181184. J Antibiot. 1992;45:1512–1517. doi: 10.7164/antibiotics.45.1512. [DOI] [PubMed] [Google Scholar]
- 19.Patterson T F, Kirkpatrick W R, McAtee R K. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. The activity of pradimicin (BMS-181184) in experimental invasive aspergillosis, abstr. B51; p. 31. [Google Scholar]
- 20.Restrepo M, Najvar L, Bocanegra R, Luther M, Graybill J. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Comparison of the efficacy of BMS-181184 (BMS) and amphotericin B (AMB) against hematogenous Candida tropicalis (CT) infection in immunosuppressed mice, abstr. F183; p. 131. [Google Scholar]
- 21.Sawada Y, Numata K, Murakami T, Tanimichi H, Yamamoto S, Oki T. Calcium-dependent anticandidal action of pradimicin A. J Antibiot. 1990;43:715–721. doi: 10.7164/antibiotics.43.715. [DOI] [PubMed] [Google Scholar]
- 22.Walsh T J, Bacher P, Pizzo P A. Chronic silastic central venous catheterization for induction, maintenance, and support of persistent granulocytopenia in rabbits. Lab Anim Med. 1988;38:467–470. [PubMed] [Google Scholar]
- 23.Walsh T J, Hiemenz J W, Anaissie E. Recent progress and current problems in treatment of invasive fungal infections in neutropenic patients. Infect Dis Clin North Am. 1996;10:365–400. doi: 10.1016/s0891-5520(05)70303-2. [DOI] [PubMed] [Google Scholar]
- 24.Yamaoka K, Nakagawa T, Uno T. Application of Akaike’s information criterion in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978;6:165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]