Figure 1. (A) Plk2-dependent degradation screen of synaptic cellular adhesion molecules.
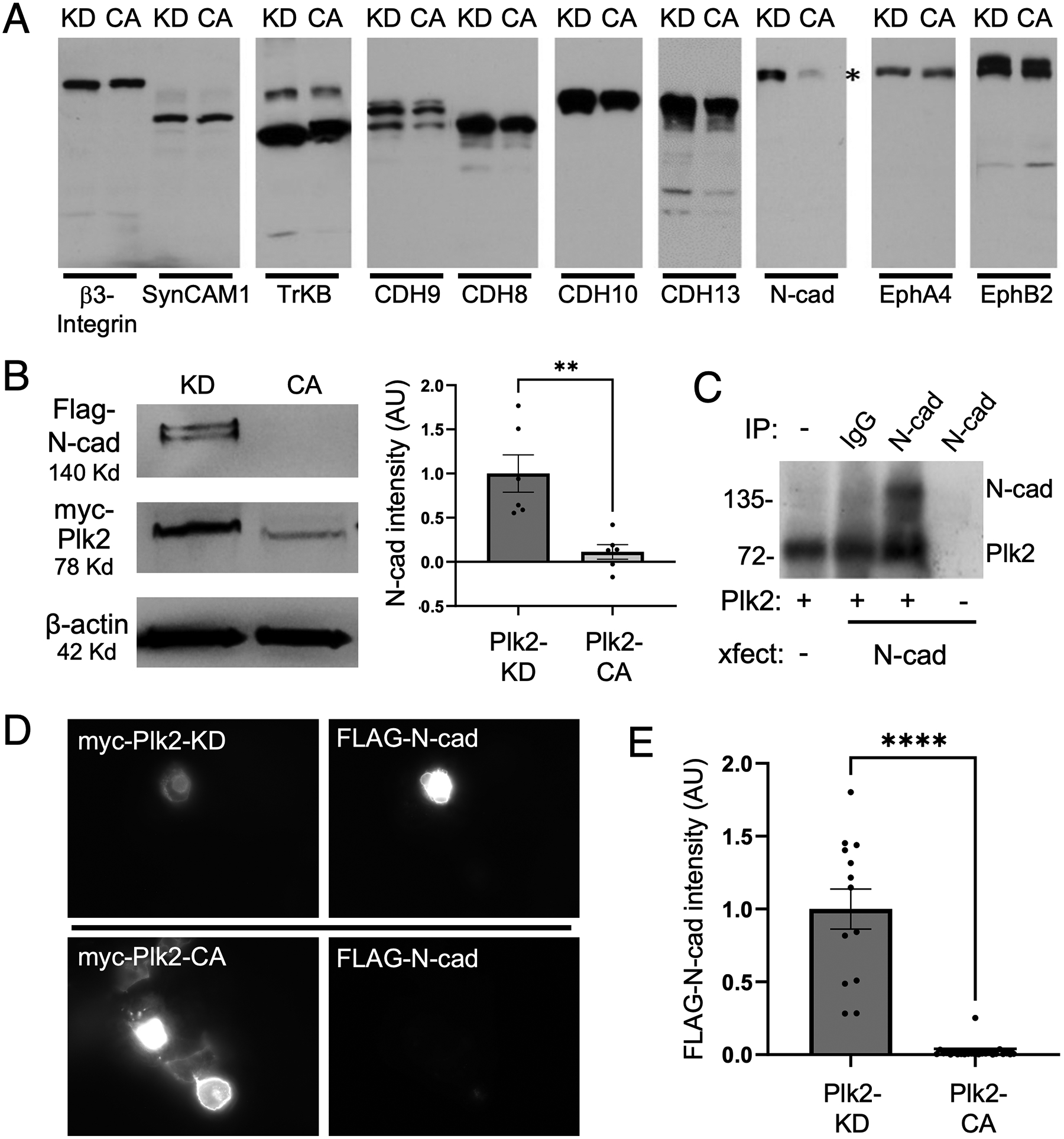
Candidate cellular adhesion molecules (CAMs) as indicated above the blots were transfected into COS-7 cells together with either kinase dead (KD) or constitutively active (CA) Plk2. Immunoblotting for each CAM demonstrated that only N-cadherin levels (highlighted by asterisk) were decreased by active Plk2. (B) Immunoblots of FLAG-N-cadherin and myc-tagged Plk2-KD or -CA co-transfected in COS-7 cells. Loading control is β-actin. Quantification is normalized to Plk2-KD; **p=0.0030, t=3.902, DF=10, unpaired two-tailed student’s t-test (n=6 cell culture preparations). (C) Direct phosphorylation of N-cadherin by Plk2. Autoradiogram of in vitro kinase assays using N-cadherin immunopurified from transfected COS-7 cells added to recombinant Plk2. Nonimmune IgG negative control to demonstrate immunoprecipitation specificity. Note Plk2 is autophosphorylated (lower band). (D) Immunostaining of COS-7 co-transfected with FLAG-N-cadherin and either myc-Plk2-KD (upper panels) or -CA (lower panels). (E) Quantification of images in (D) normalized to Plk2-KD; ***p<0.0001, t=9.702, DF=35, unpaired student’s t-test (n=13–24 cells).
