Figure 7. Summary of mutation effects on biological process of Protein C.
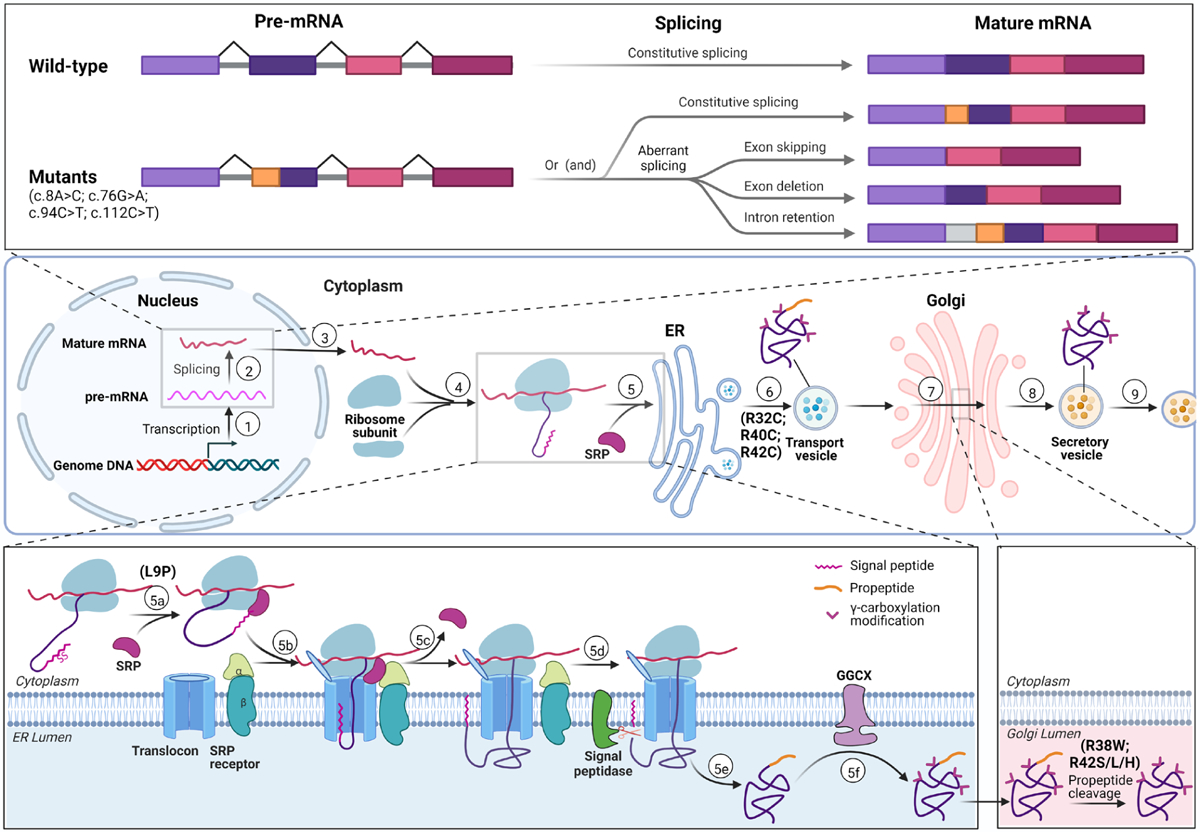
Top panel: Diagram of variants affecting pre-mRNA splicing. Mutations (c.8A>C, c.76G>A, c.94C>T, c.112C>T) can lead to aberrant splicing, including exon skipping, exon deletion and intron retention. The orange color indicates the region contains the variation. Middle panel: Scheme of PC biological process from gene transcription to protein secretion. In nucleus, the PROC gene is transcribed (①) into preliminary mRNA (pre-mRNA), which is spliced into mature mRNA (②); Mature mRNA transfers to cytosol (③) and forms translation initial complex with ribosome subunits (④). Directed by SRP, the translation initial complex is translocated to ER to finish translation, and then the protein is modified in ER lumen (⑤). The protein is transferred to Golgi complex by transport vesicle (⑥), and further modification (including propeptide cleavage) is processed to generate mature PC in Golgi (⑦). Mature PC is coated into secretory vesicles (⑧) and secreted into extracellular space (⑨). Mutations of R32C, R40C, R42C may interfere the step ⑥ and lead to ER retention. Bottom panel: Biological process of protein C in ER and Golgi. Bottom left: Biological process of PC from cytosol to ER. SRP recognizes the signal peptide (5a), directs the translation complex to translocon in ER membrane to continue translation (5b), and then is released (5c). The signal peptide is cleaved by signal peptidase (5d). After translation is finished (5e), PC is translocated into ER lumen, in which γ-carboxylation is carried by GGCX. L9P mutation destroys the signal peptide function which translocates protein translation from cytosol into ER by binding SRP. Bottom right: PC propeptide is cleaved by propeptidase in Golgi complex. Mutations of R38W and R42S/L/H lead to mis-cleaved or uncleaved propeptide in mature PC.
