Figure 2. Intravenous heterologous prime-boost vaccination elicits high-magnitude CD8 T cell responses that protect mice from tumor challenge.
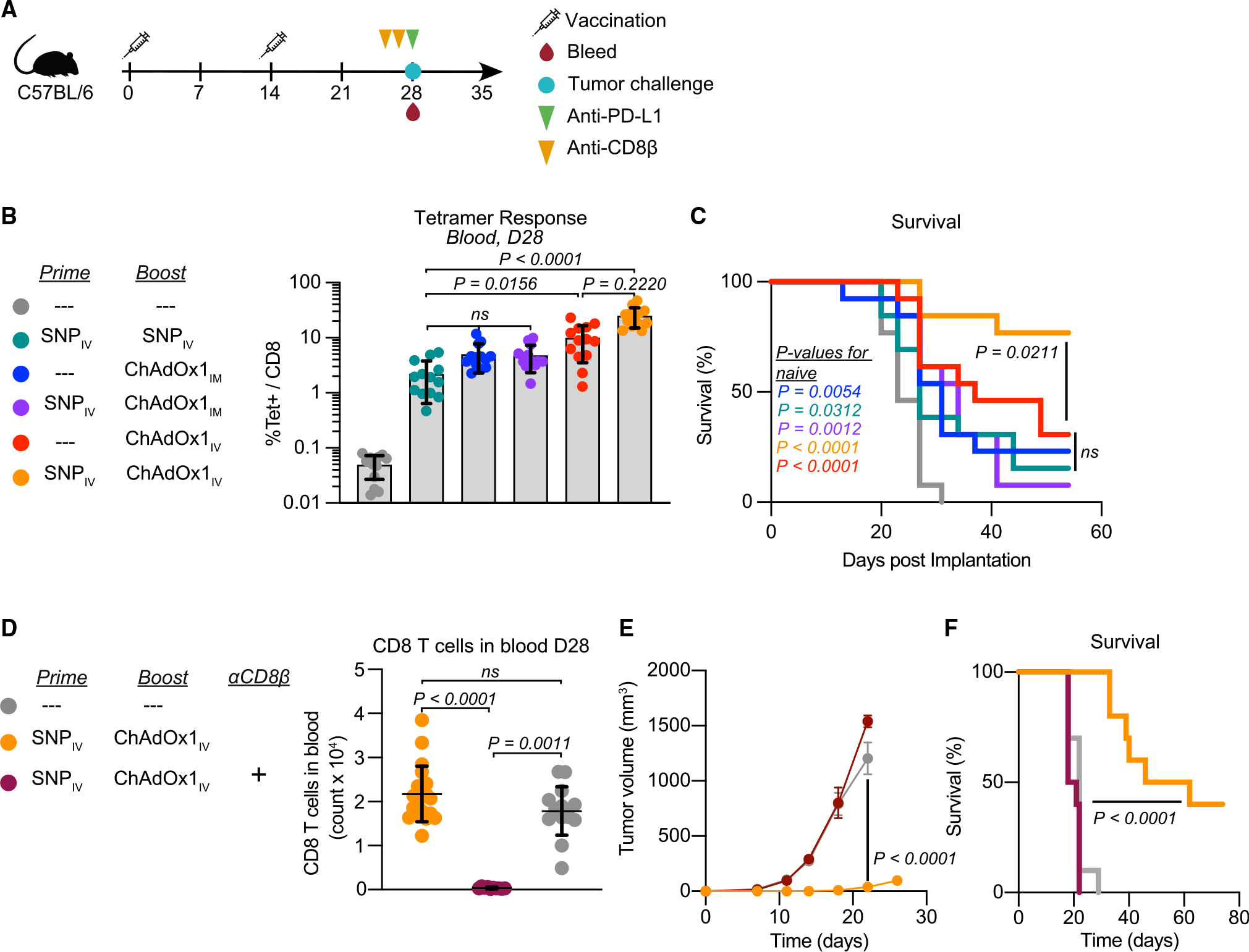
(A) Prophylactic study vaccination and sampling schedule. Mice are primed and boosted 2 weeks apart and then challenged with tumor cells 2 weeks post-boost. At the time of tumor challenge, mice are also bled to assess T cell responses and given 1 dose of αPD-L1. Some mice also received αCD8β antibody 3 days and 1 day prior to tumor challenge.
(B and C) Magnitude of Reps1-specific CD8 T cell responses in blood at the time of tumor challenge measured by tetramer staining (B). Survival curve following tumor implantation (C).
(D–F) CD8 T cell count in blood at time of challenge (D). Average tumor growth curves following MC38 tumor challenge in i.v. heterologous prime-boost group with and without CD8 T cell depletion (E). Survival curve for i.v. heterologous prime-boost group with and without CD8 T cell depletion (F).
In (B) and (D), data represented as mean ± SD; Kruskal-Wallis test with Dunn’s correction for multiple comparisons. In (C) and (F), Mantel-Cox log rank test, compared with naive mice unless otherwise indicated. In (E), two-way ANOVA with Bonferroni correction for multiple comparisons. In (B) and (C), n = 13 and representative of 2 replicates; in (D)–(F), n = 10, 1 experiment.
