Fig. 3. Adenovirus-mediated knockdown of hepatic SRPK2 ameliorates chronic-binge alcohol feeding-induced fatty acid synthesis, steatosis, and liver injury in mice.
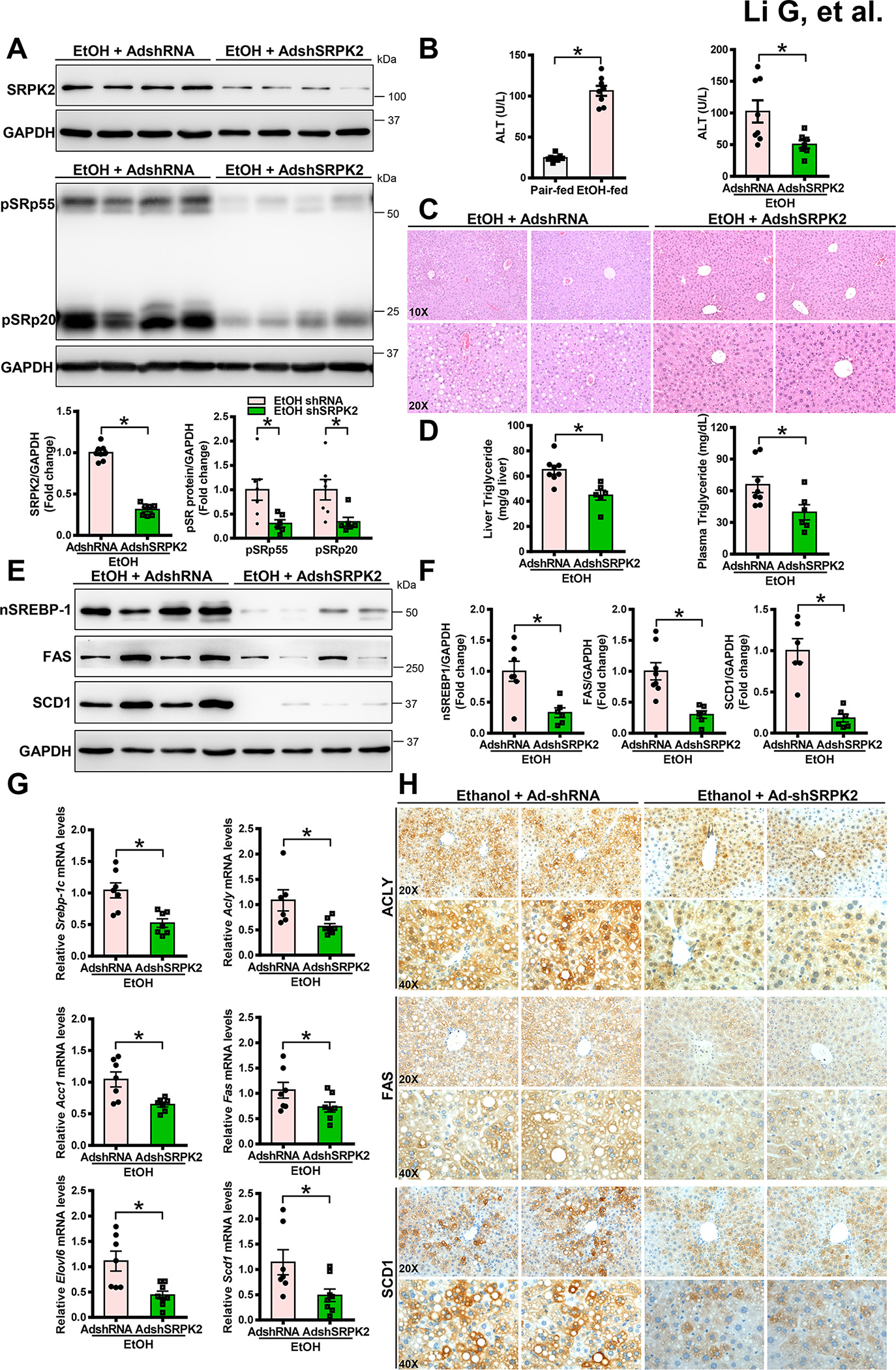
Mice were administered via tail vein injections of adenoviral vectors encoding shRNA targeting the SRPK2 gene (AdshSRPK2) or shRNA control (AdshRNA). After injection, the mice were fed a Lieber-DeCarli alcohol liquid diet for 10 days, plus one binge of alcohol at the end of experiments. All mice were subsequently sacrificed 9 hours post-binge.
A. Immunoblots and densitometric quantification for the abundance of SRPK2 and phosphorylation of SR proteins. Adenovirus-mediated knockdown of hepatic SRPK2 in mice was confirmed by a dramatic decrease in SRPK2 levels and SR protein phosphorylation.
B. Alcohol-induced elevation of liver injury, as assessed by plasma ALT levels, was reduced in mice upon SRPK2 knockdown.
C. H&E staining for hepatic steatosis in mice.
D. Chronic-binge alcohol feeding-induced elevation of hepatic and plasma triglyceride levels in mice were lowered by silencing SRPK2.
E-F. Immunoblots (E) and densitometric quantification (F) for nSREBP-1, FAS, and SCD1.
G. Real-time qRT-PCR analysis of hepatic lipogenic gene expression. Ad-shSRPK2-injected mice exhibited downregulation of SREBP-1c and its target genes under conditions of chronic-binge alcohol feeding.
H. Immunohistochemistry staining of liver sections using antibodies against ACLY, FAS, or SCD1. Upon chronic-binge alcohol feeding, strong positive staining for ACLY, FAS, and SCD1 was visualized mainly in hepatocytes of Ad-shRNA control-injected mice; however, the number and intensity of ACLY+, FAS+, and SCD1+ hepatocytes were reduced by silencing hepatic SRPK2.
The data are presented as the mean ± S.E.M., n=6–8 per group. *P<0.05 between two groups.
Images were acquired using 20X and 40X objectives.
