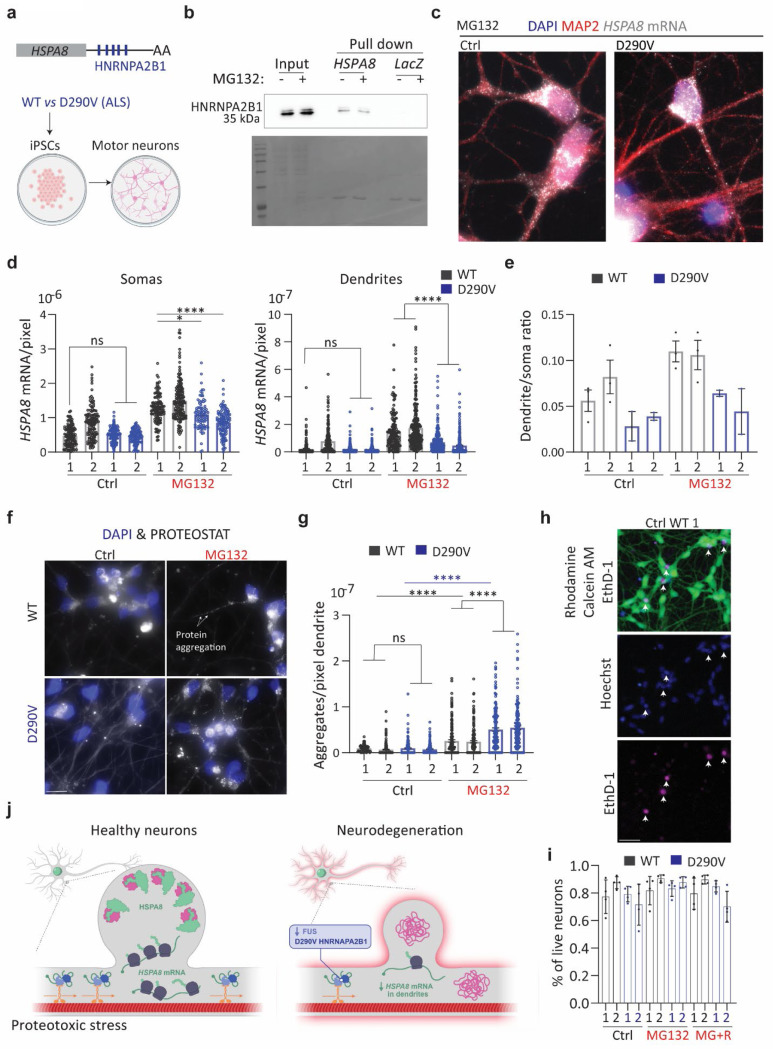
Fig 7. An ALS-associated HNRAPA2B1 mutation impairs dendritic HSPA8 mRNA localization in human motor neurons.
(a) Schematic of human HSPA8 mRNAs and the differentiation of iPSCs from healthy and D190V donors into motor neurons. (b) Pulldown experiments to validate the binding of HNRNPA2B1 to the HSPA8 3′ UTR were analyzed by western blot. I, input; PD, pulldown; low and high refer to different exposure times of the same blot. (c) IF-smFISH to stain dendrites with an anti-MAP2 antibody and detect HSPA8 mRNAs in MG132-stressed motor neurons differentiated from healthy (WT) donors and patients with ALS carrying the HNRNAPA2B1D290V mutation. Scale bar = 10 μm. (d) Quantification of somatic and dendritic HSPA8 mRNAs in Ctrl and MG132-stressed human-derived motor neurons from the experiments in C. Data are the mean ± SEM of two independent experiments (n = 84–155 neurons, n = 150–331 dendrites individual soma and dendrite values indicated by a dot). Motor neurons differentiated from healthy donors WT and patients (P). ***, P < 0.001; **, P < 0.01; *, P < 0.05; ns, no significant (by 1 way ANOVA). (e) The ratio of HSPA8 mRNA per pixel of soma or dendrite area in the MG132-stressed motor neurons analyzed in C. (f) Representative images of protein aggregates detection in WT and D290V HNRNPA2B derived motor neurons stress with 10 μM MG132 for 7 h. (g) Quantification of the aggregates detected by the PROTEOSTAT staining in dendrites in F. Data are the mean ± SEM of three independent experiments (n = 42–59 dendrites). ****, P < 0.0001; ns, no significant (by 1 way ANOVA). (h) Representative neurons from WT MG132-stress iPSCs-derived motor neurons double-stained to identify live (Calcein, green) and dead (EthD-1, magenta) neurons and nuclei (Hoechst, blue). Arrowheads indicate dead neurons in the three channels. Scale bar = 10 μm. (i) Quantification of the live neurons in Ctrl, MG132-stress (10 μM for 7h), and recovery (10 μM MG132 for 7h and 4 h after MG132 washout). Four independent experiments (n = 10 fields of view). ns; no significant (by Wilcoxon test). (j) Summary of conclusions. Neurons sustain dendritic proteostasis in response to stress by increasing the localization of HSP mRNAs, mainly Hspa8, and their translation. The regulated transport of Hspa8 mRNA is mediated by two RBPs, FUS and HNRNPA2B1. Depletion of FUS or expression of the ALS mutation HRNAPA2B1D290V reduces the localization of Hspa8 mRNAs and promotes the accumulation of misfolded proteins upon stress. Dendritic attrition and loss of synaptogenesis are common signs of diverse neurodegenerative diseases, and defects in dendritic HSP mRNA localization offer a molecular explanation for these events.