Abstract
Extensive research has focused on the compositional changes in dental plaque microbiome communities during the transition from health to disease, known as dysbiosis. However, alterations in the spatial composition of these communities throughout the progression from health to disease remain under-explored. We describe an in vitro dental plaque model for culturing oral biofilms seeded with dental plaque from human volunteers. Our model recapitulates important features of the in vivo environment including shear force induced by salivary flow over teeth and the nutritional milieu experienced by microbes that inhabit the transitional zone between supragingival and subgingival aspects of the teeth. Importantly, our model is amenable to multiplex fluorescent labeling and multispectral imaging for testing specific hypotheses regarding systems-level community structure and function. The model allows for precise manipulation of various environmental conditions, such as flow rate and nutrient availability to investigate their effects on biofilm development and spatial structure. Furthermore, this model can be used to test the effects of various therapeutic interventions, such as antimicrobial agents, on the biofilm composition and structure at the micron to millimeter scale, making it a valuable tool for studying the molecular and cellular basis of dental plaque-mediated diseases and for benchmarking new therapeutic interventions.
Basic Protocol 1:
Dental plaque on a chip in vitro model culture system
Support Protocol 1:
Gingival Margin (GM) medium preparation
Basic Protocol 2:
Microcosm Labeling and Multispectral Image Acquisition
Keywords: Biofilms, Oral Microbiology, Dental Plaque, In Vitro Models, Spectral Imaging
INTRODUCTION:
Dental plaque biofilms are complex microbial communities that mediate oral health and diseases such as dental caries and periodontal disease. Despite extensive research, there remains a significant gap in understanding how changes in spatial structure of resident microbial communities impact oral disease progression. Sequencing based approaches to studying oral microbial communities provide information on taxonomic composition with high breadth but provide limited spatial information at the micron scale. Synthetic polymicrobial biofilms composed of oral isolates offer a high degree of control and are compatible with in situ imaging based approaches but lack the complexity of naturally derived communities.
We present a method for growing highly complex, in vitro dental plaque biofilms and performing downstream multiplex labeling and multispectral imaging. Multispectral imaging allows for the simultaneous acquisition of multiple fluorescent signals, offering micron-scale analysis of the spatial distribution of developing oral microbial communities, in situ. This approach allows for investigation into the spatial dynamics of oral biofilm formation, the biophysical and biochemical interactions among different bacterial species, and the response of the biofilm to various treatments under controlled laboratory conditions.
Here we describe the setup and growth of oral biofilms seeded with supragingival plaque of healthy volunteers with an in vitro flow cell culture model (Basic Protocol 1) (Fig. 1). We then provide instructions for harvesting, labeling, multispectral imaging, and linear unmixing (LU) of in vitro dental plaque biofilms (Basic Protocol 2). LU can be achieved with various commercial implentations included with microscope manufacturers' software, a free-ware plugin for ImageJ or custom algorithms previously described in the literature (Gammon et al., 2006; Wang et al., 2022; Zimmermann et al., 2013). We present a procedure for acquiring multispectral images of model biofilms, which are labeled using one domain level and 7 genus-level fluorescence in situ hybridization (FISH) probes. In principle, this approach can be adapted to a wide variety of biofilm models of clinical relevance provided taxon specific probes are available.
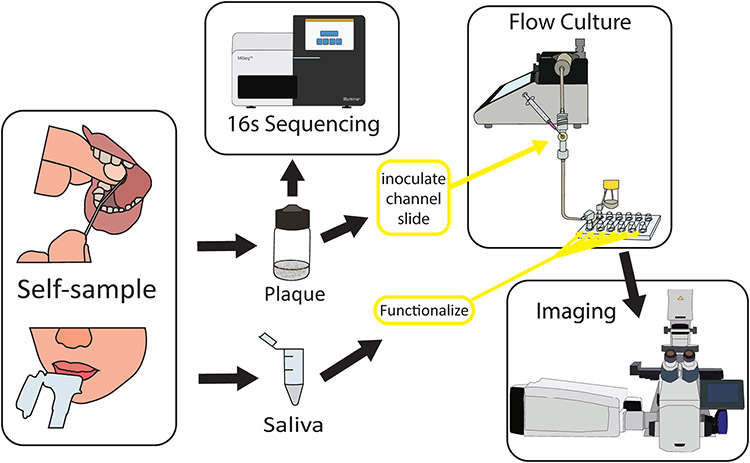
Figure 1:
Overview of experimental procedure for in vitro dental plaque model. Dental plaque is self-collected by a healthy volunteer. In the laboratory, the sample is aliquoted and sent for DNA sequencing. Collected saliva is used to functionalize a flow-cell microslide. A flow system is constructed, and media is pumped via a syringe pump over the functionalized slide. The culture is inoculated with dental plaque through an injection port upstream of the slide and the system is incubated at 37 C. Biofilms are fixed and labeled for multispectral imaging.
CAUTION: All reactions must be run in a suitable fume hood with efficient ventilation. Many of the reactions in this article involve the use of teratogens and mutagens; safety glasses and reagent-impermeable protective gloves should be worn.
CAUTION: Many isolated species from human dental plaque are Biosafety Level [2] ([BSL-2) pathogens. Follow all appropriate guidelines and regulations for the use and handling of pathogenic microorganisms.
STRATEGIC PLANNING
The need to work quickly when volunteers arrive to submit dental plaque samples is necessary to preserve the microbial communities present in the dental plaque. Many dental plaque microbes are obligate anaerobes. Cryo-freezing medium should be pre-reduced and stored anaerobically before time of collection. Additionally, careful selection of fluorophores is vital for successful multispectral imaging (see Cohen, et al. for a discussion of fluorophore selection(Cohen et al., 2018; Zimmermann, 2005)). In this protocol, we describe the creation and use of 8 reference images, each showcasing singly labeled Escherichia coli cells, aligned with the methodology presented by Valm et al. (2011) in their CLASI-FISH technique. Each of these images corresponds to a specific fluor that will be used in the final sample, and they serve the crucial purpose of providing reference spectra for the linear unmixing process.
We utilize then use Zen Blue software which includes an integrated LU function for viewing multispectral images and extracting reference spectra. In planning to conduct research using this in vitro model it is important to create a singly labeled sample for each fluorophore that will be present in the experimental sample.
BASIC PROTOCOL 1: DENTAL PLAQUE ON A CHIP IN VITRO MODEL CULTURE SYSTEM
This protocol outlines a systematic approach for the study of oral biofilms using an in vitro dental plaque-on-a-chip flow cell model. The initial phase involves the recruitment of healthy donors who are instructed to abstain from oral hygiene practices for 48 hours. This period of abstention allows for the accumulation of a representative dental plaque sample. From each participant, both a saliva and dental plaque sample will be collected and preserved (stored at −80C) for later use within the flow cell model, simulating conditions conducive for biofilm formation. We introduce a support protocol for making gingival margin medium (GMM), a media that is used in our flow cell model that mimics the nutrient conditions of the oral environment. At the conclusion of the culturing process, the resultant sample will contain a diverse oral microcosm ready for preservation and multispectral labeling in Basic Protocol 2.
Dental plaque samples that are used to seed the in vitro model system are obtained from healthy volunteers through dental flossing. Volunteers are instructed to abstain from oral hygiene (i.e., tooth brushing, flossing, and using mouthwash) for 48 hours, after which informed consent is confirmed, and samples are collected by laboratory personnel. Volunteers are asked to floss between 12 teeth with 12 separate pieces of dental floss and subsequently expectorate 10 mL of saliva into a sterile collection tube. Each piece of floss containing dental plaque is split into two aliquots and immediately stored at −80 °C for future use. Saliva collected from volunteers is centrifuged at 8000 rcf and stored at −20 °C for later flow cell hybridization. Dental plaque and saliva can be stored for up to one year before use.
The support protocol described here, including inclusion and exclusion criteria was adapted from U.S. National Institutes of Health Human Microbiome Project, Manual of Procedures Core Microbiome Sampling Protocol A: HMP Protocol # 07-001, version 12.0 accessible at https://www.hmpdacc.org/hmp/ (Methé et al., 2012).
CAUTION: The use of human subjects as described in this protocol was approved by the State University of New York at Albany Institutional Review Board (IRB), Human Subjects Protocol Nos.: 21E127 and 21E128. Before working with human subjects, researchers should contact the applicable regulatory bodies for approvals including those of the research institution and funding agencies. Laboratory personnel should receive proper training in working with human subjects according to the regulations of their institution.
Equipment
Human Volunteer Dental Plaque Collection
Benchtop microcentrifuge
Benchtop swinging bucket centrifuge
Anaerobic chamber
Class II biological safety cabinet
Cryofreezer
Dental Plaque on Chip Model
6-10 Channel Syringe Pump (e.g., Cole Parmer cat. no. 78-8200C)
Gravity Incubator
Class II Biological Safety Cabinet
Materials:
Human Volunteer Dental Plaque Collection
Ice bucket with ice
Sterile gloves
50 mL conical tube (VWR Cat. No. 82050)
1.5 mL microcentrifuge tube (VWR Cat. No. 10025)
Sterile PBS solution (Fisher Cat. No. 10010023)
Dental floss (Benco Dental Cat. No. 4799-917)
Bottled potable water
Pre-reduced Liquid Dental Transport (LDT) media (Anaerobe Systems Cat. No. AS-916)
Anaerobic glycerol (Fisher Cat. No. 35-635-2100ML)
2 μm filters (e.g., Fisher Cat. No. 720-1320)
50 mL syringe (e.g., VRW Cat. No. BD309653)
15 mL conical tube
Screw cap silicone gasket cryovials (e.g., Fisher Scientific Cat. No. 10-269-88A)
Dry ice
>99% ethanol
Dental Plaque on Chip Model
1x μ-Slide VI 0.5 Glass Bottom (Ibidi, cat. no.: 80607)
2x Elbow Luer Connectors (Ibidi, cat. no.s: 10802, 10826)
1x Luer Lock Connector, In-line Luer Injection Port, Luer Lock Coupler (Ibidi, cat. no.s: 10826, 10820, 10823)
0.8 mm Silicone Tubing (Ibidi, cat. no.: 10841)
1x 20 ml Luer-Lok Syringe sterile (e.g., BD, cat. no.: 302832)
3x 1 ml Syringe with Needle (e.g., BD, cat. no.: BD 309597)
Dental plaque and saliva from donor
150 mL media bottle for waste receptacle.
Mineral salt buffer (see recipe in Reagents and Solutions)
Gingival Margin (GM) Medium (see Support Protocol 1)
Protocol steps with step annotations
Human Volunteer Dental Plaque Collection
Preparation of 50% anaerobic glycerol-dental transport medium (DTM)
Transport 500 mL glycerol bottle, 2 μm filters, 50 mL syringe and ~25 x 1 mL bottles of LDT to the anaerobic chamber. Inside the chamber, loosen the cap on the glycerol bottle.
Using a pipettor, transfer 20 mL of LDT to a 50 mL conical tube. Carefully pour 20 mL of glycerol to the tube. Vortex the tube vigorously to suspend the glycerol.
Place a 2 μm filter on the end of a 50 mL syringe.
Pour the 50% glycerol mixture into the syringe.
-
Use the plunger to force the 50% glycerol solution through the filter. Collect in a sterile filter cap 50 mL tube. Invert the tube 3 x, then vortex for 60 seconds to mix. Keep this mixture in the anaerobic chamber for long term use.
This medium should be prepared up to 6 months before collecting oxygen-sensitive dental plaque and saliva samples and kept in the anaerobic chamber until use.
Volunteer Sample Collection and storage
-
Potential volunteers telephone the laboratory to provide verbal informed consent to participate in the research protocol.
This step is required because volunteers begin their participation 48 hours before visiting the lab, over which period they abstain from the following oral hygiene procedures: tooth brushing, flossing, and use of antiseptic mouthwashes.
On the day of the human volunteer visit, go over the study design with the volunteer, obtain signed informed consent to proceed with the protocol and provide a physical copy of the informed consent document to the volunteer. Confirm inclusion and exclusion criteria, namely that the volunteer has abstained from oral hygiene for the previous 48 hours, the volunteer does not normally require prophylactic antibiotics before dental procedures to prevent Infectious Endocarditis (IE), and that the volunteer has not knowingly taken any prescription antibiotics in the 6 months before their visit.
Instruct the volunteer to floss between 12 pairs of teeth, (3 pairs of teeth in all 4 quadrants) and deposit each piece of floss in a sterile 1.5 mL microcentrifuge tube, then close the lid.
-
Instruct the volunteer to expectorate 10 mL of unstimulated saliva in a sterile 50 mL conical tube with the 10 mL mark highlighted. Provide the volunteer with bottled potable water but instruct them not to avoid spitting water into the collection tube.
The saliva tube is kept on ice during the collection procedure, which may take up to 1 hour or more depending on the volunteer.
-
After providing all instructions, leave the room to give the volunteer privacy during collection.
Immediately upon completion of collection, process plaque and saliva samples for long-term storage.
Cryopreservation of dental plaque samples
Transport the 12 dental floss tubes into the anaerobic chamber.
To the first tube, pipet 1 mL LDT. Pipet up and down vigorously 20 times to dislodge all the dental plaque from the floss. Close the lid on the tube and vortex mix for 10 s.
Open the tube, remove the floss and discard in a dedicated autoclave waste bag.
Transfer the 1 mL of plaque solution to the next dental floss tube and repeat step 3 until you have resuspended 6 tubes.
After 6 tubes, start over with a fresh 1 mL of LDT and collect the plaque from the remaining 6 tubes.
Combine both plaque suspensions into one 15 mL conical tube.
Pipet 2 mL dental transport medium to the plaque suspension to create a 25% glycerol solution. Vortex mix for 5 s.
Aliquot 200 μL of this glycerol plaque suspension into each of 20 screwcap, silicone gasket cryovials.
-
Screw the cap on tight on the cryovials and remove from chamber.
It is important to screw the cap and form a tight seal to prevent both oxygen and ethanol from entering the tube in subsequent steps.
-
Prepare a dry ice - ethanol bath.
Prepare bath in the chemical fume hood and wear appropriate personal protective gear including lab coat, cryo-gloves, and face shield to prevent supercooled ethanol from contacting skin.
-
Wait 10 minutes for the dry ice-ethanol bath to reach a low boil. Drop each of the 20 tubes in a test tube rack in the dry ice ethanol bath. Let them sit there for 5-10 minutes to snap freeze.
Make sure the tubes are not fully submerged in the ethanol bath. The cap should be above the ethanol to prevent contamination of the sample with ethanol.
After 10 minutes, remove the cryovials carefully using forceps and place in a freezer box. Place the box in a −80 °C cryofreezer for storage up to 1 year.
Cryopreservation of saliva cells and preservation of saliva
-
In a bench-top swinging bucket centrifuge, spin the 50 mL saliva tube at 12,000 RPM for 20 minutes at 4C.
[*Copyeditor: Please ask the authors to provide the rcf unit for the centrifugation step above]
Transport the 50 mL tube containing human saliva to the biological safety cabinet.
-
Open the tube and decant the saliva into a sterile 50 mL centrifuge tube. Use caution, the separated saliva may stick tightly to the cell pellet.
If the cell pellet accidentally gets disturbed, recombine pellet with supernatant and spin it again for 20 min in centrifuge.
Store cell-free saliva at −20 °C for up to 1 year.
Transport the saliva cell pellet to the anaerobic chamber.
Resuspend the pellet in 2 mL LDT. Pipet up and down vigorously 20 times. Vortex mix for 10 s.
Add 2 mL dental transport medium. Pipet up and down 10 times and vortex mix for 5 s.
Aliquot 200 μL of the cell pellet glycerol slurry into each of 20 screwcap cryovials. Screw the cap with silicone gasket on tightly.
Drop each of the 20 tubes in the dry ice ethanol bath prepared as described previously. Allow the pellets to freeze solid in the bath.
After 20-30 minutes, remove the cryovials carefully using forceps and place in −80 °C cryofreezer for long-term storage.
Dental Plaque on Chip Model Assembly and Growth Flow Cell Assembly
Attach female Luer lock connectors to each end of a 10-inch section of 0.8 mm silicone tubing (Fig. 2).
Attach the In-line Luer Injection port to the female Luer lock connector on the compatible end.
Attach the male Luer lock connector to the opposite end of the In-line Luer Injection port.
Attach a 2-inch section of silicone tubing to the male Luer lock connector.
Attach the male elbow Luer lock connector to the opposite side of the 2-inch silicone tubing.
Attach female Luer lock coupler to end of male elbow Luer lock connector.
Attach the remaining male elbow Luer lock connector to the other section of the 10-inch silicone tubing.
Complete the line by attaching the male elbow Luer lock connector to the open end of the female Luer lock coupler.
Place the end of the tubing assembly without the female Luer lock connector into the waste receptacle.
-
Draw 20 ml of Gingival Margin (GM) media into 20 ml BD Luer-Lok sterile syringe and attach to female Luer lock connector
Ensure air bubbles are removed from the media syringe prior to assembly.
Place the syringe pump in a 37C incubator.
-
Insert syringes (with tubing assembly complete) into pump setup placed in 37 °C incubator and set the flow rate to 5 μL/min.
Placing the syringe pump setup above the flow cell and starting flow immediately (before adding Ibidi μ-Slide) helps prevent bubbles in the system.
In the biological safety cabinet, dilute donor saliva 1:1 with sterile DI water (total volume x mL) and spin down at 3220 RCF in a bench top swinging bucket centrifuge for 10 minutes to remove cells
-
Pipette 50 μl of dilute donor saliva into Ibidi μ-Slide flow channel and incubate for 1 hour at room temperature under U.V. light with sash closed in a biological safety cabinet.
This step functionalizes the coverslip surface in the μ-slide with salivary proteins and glycoproteins to form an adhesive surface for early colonizing plaque species.
In the 37 °C incubator, detach the male elbow Luer lock connectors from the female Luer lock coupler of running flow setup and securely attach to the Ibidi μ-Slide flow channel inlet followed by outlet (Fig. 3).
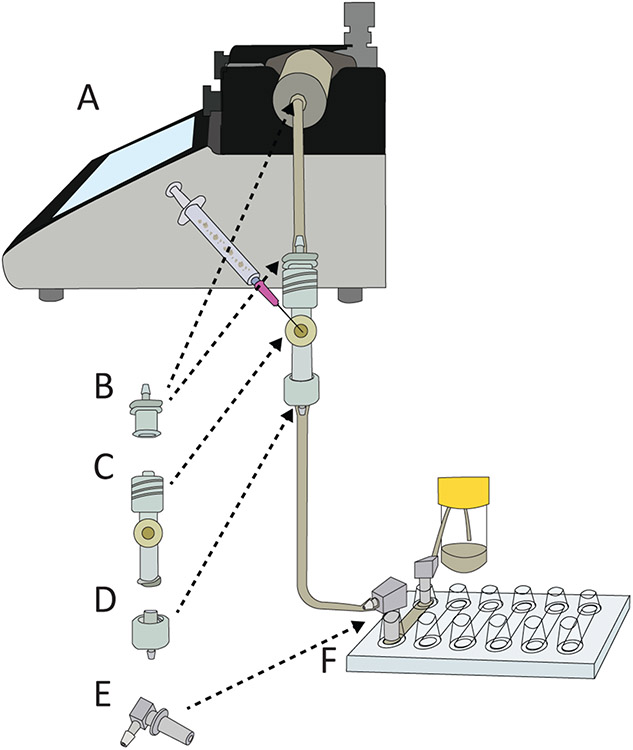
Figure 2:
Diagram of dental plaque on a chip flow cell setup. (A) Syringe pump for precise control of flow rate (B) Female Luer Lock Connector, (C) injection port (D) male luer lock connector (E) elbow luer lock connector, and (F) Ibidi multichannel microslide.
Figure 3:
Image of in vitro dental plaque on a chip culture model. Six media containing syringes are loaded into a multichannel syringe pump set on a shelf above the multichannel slides. Each flow slide has three channels developing biofilms derived from the supragingival plaque of a single healthy donor. Flow slides are placed on a slight incline rising towards waste receptacle to prevent bubble accumulation.
Inoculation and Microcosm Culture
-
16.
Rapidly thaw donor dental plaque (stored at −80C) in a 37 °C water bath and spin down at 8000 RCF for 5 minutes in a benchtop microcentrifuge to gently remove freezing medium, followed by 2x washes in mineral salt buffer. Rehydrate washed dental plaque in 50 μl of mineral salt buffer for each biofilm to be grown (e.g., for six biofilms, rehydrate in 300 μl of mineral salt buffer).
Do not spin live cells at higher speed than 8000 RCF, this could damage the cells and differentially reduce viability.
-
17.
Using a small binder clip clamp the tubing upstream of the injection port. Draw 50 μl of dental plaque solution into a sterile syringe and inject into the port, then remove the clamp to restart flow.
Clamping the tubing line upstream of the injection port during plaque inoculation prevents dental plaque contamination of the GM media upstream of the inoculation port.
-
18.
Repeat steps 16 & 17 at 3 hours and 6 hours for a total of three inoculations.
SUPPORT PROTOCOL 1: Gingival Margin (GM) Medium Preparation
GM medium is adapted from Shi medium, a fortified medium capable of culturing in vitro supragingival biofilms closely resembling the diversity of the initial inoculum (Tian et al., 2010). GM medium is modified to better recapitulate the nutritional milieu experienced by microbes at the gingival margin (transitional zone). Sheep's blood is replaced with heat-inactivated human serum. To induce inter-cellular metabolic cooperation, components of Shi media that presumably are derived not from the host but rather from other members of the microbiota, i.e., N-acetyl muramic acid and vitamin K are not included and the final media is diluted to 50% strength in DI water. After medium preparation is complete, the media should be filter sterilized to remove large mucin particles which non-specifically bind and sequester FISH probes and interfere with downstream quantitative image analysis.
Materials:
sterile 10 mL syringe (e.g., BD 301029)
sterile 0.2 μm PES syringe filter (e.g., Corning 431229)
150 mL sterile disposable 0.2 μm PES filter unit (e.g., Thermoscientific cat. no. 5650020)
Hemin Solution
Hemin (Sigma, cat. no.: 51280)
K2HPO4 (Fisher, cat. no.: P288-50)
MiliQ H2O
Base Media
Protease peptone (Fisher, cat. no.: BP1420-500)
Trypticase peptone (BD Bacto, cat. no.: 211705)
Yeast extract (BD Bacto, cat. no.: 212750)
Porcine gastric mucin (Sigma, cat. no.: M1778)
KCl (Fisher, cat. no.: P2175)
Urea (Fisher, cat. no.: U15-500)
L-arginine (Fisher, cat. no.: BP370-100)
L-lysine (Fisher, cat. no.: BP386-100)
Glycine (Fisher, cat. no.: BP381-500)
Sucrose (Fisher, Cat. No. A15583-36)
Heat Inactivated Human Serum (Sigma, cat. no.: H3667)
Note: Serum should be stored at −20 C and only be added to the media at the time of the experiment.
Protocol steps with step annotations
GM media preparation protocol
Prepare hemin solution in advance by combining 10 mg Hemin, 1.74 g K2HPO4, and 100 ml MiliQ H2O, in flask and heat to boiling. Store in 10ml aliquots at −20 C.
Prepare the base media stock by combining 10 g protease peptone, 3 g trypticase peptone, 5 g yeast extract, 2.5 g porcine gastric mucin, 2.5 g KCl, 0.06 g urea, 0.87 g L-arginine, 0.182 g L-lysine, 0.075 g glycine, and 1.0 g sucrose in a 2 L flask followed by the addition of 970 ml of DI H2O and add 10 ml of Hemin solution.
Dilute the media 1:1 in DI H2O and autoclave at 121 °C for 45 minutes. Allow to cool to below 60 °C.
Filter sterilize media to remove large mucin particles in a 150 mL filter unit and store at 4 °C until needed.
Thaw and filter sterilize 6 mL of human serum using a 10 mL syringe with a 0.2 μm filter attached. Add the filter-sterilized human serum to 120 mL of GM media, resulting in a 20:1 ratio of GM media to human serum.
Warm the mixture of GM media and human serum to 37 °C just prior to use.
BASIC PROTOCOL 2: Microcosm Labeling and Multispectral Image Acquisition
The Microcosm Fixation and FISH Labeling Protocol delineates a method for the fixation of dental plaque biofilms and their subsequent labeling using Fluorescent In Situ Hybridization (FISH). This approach allows for the detailed examination of spatial distribution and identification of microbial taxa within biofilm communities. Coupled with multispectral imaging, the protocol enables a comprehensive characterization of biofilm structures and their constituent members, providing insights essential for understanding microbial dynamics in dental plaque biofilms.
CAUTION: Always handle HiDi formamide inside a certified fume hood due to its teratogenic properties. Ensure proper personal protective equipment is worn and dispose of waste in accordance with institutional and local regulations.
Equipment
Hybridization oven capable of adjustable temperature in range of 46-48 °C
Laser scanning confocal microscope equipped with spectral detector(s) (e.g., Zeiss LSM 710, 880, 980; Nikon A1; Leica SP5, SP8; and others)
Materials
Fixation
4% Paraformaldehyde (PFA) (Electron Microscopy Sciences, cat. no. 157-4)
Phosphate-buffered saline (PBS, Fisher, cat. no. 10010023)
Ethyl alcohol
0.75" paper binder clip (e.g., Staples, 10667-CC)
1 M Tris pH 7.5
1% sodium dodecyl sulfate (SDS)
HiDi formamide (Thermofisher Cat# 4440753)
Milli-Q ultrapure water
NaCl
FISH buffer (see recipe in Reagents and Solutions)
Wash Buffer 1 (see recipe in Reagents and Solutions)
Wash Buffer 2 (see recipe in Reagents and Solutions)
SlowFade Gold Antifade Mountant (Invitrogen cat. no. S36940)
Protocol steps with step annotations
Microcosm Fixation and FISH Labeling Protocol
-
Clamp silicone tubing with 0.75" binder clip directly upstream and downstream of Ibidi μ-Slide flow channel, followed by the careful removal of Luer lock elbow connector. Attach each end of Luer lock elbow connector to Luer lock coupler so that remainder of setup breakdown can be done later.
Clamping tubing prior to removal of μ-slide from flow setup helps to preserve fragile biofilm from being disturbed by mechanical stress and media flow during removal from setup.
-
Gently pipette 60 μL of 4% PFA solution into each biofilm containing flow channel, angling the pipette tip towards Ibidi μ-Slide reservoir wall. Cover with included Ibidi μ-Slide lid and allow to fix for 1.5 hours at room temperature.
Angling pipette tip towards reservoir wall serves to preserve fragile biofilm. After removal of elbow Luer lock connectors, there is approximately 60 μL of media volume left over in the channel/reservoir. The addition of 60 μL 4% PFA yields a final concentration of 2% PFA. NOTE: The total combined volume of channel and reservoir is 160 μL.
Remove approximately 60 μL of PFA solution by pipetting from channel outlet. Subsequently, wash flow channel twice by using 120 μL of PBS solution followed by twice with a 1:1 ethanol to PBS solution. Seal with male Luer lock plugs and store at 4 °C for at least 24 hours prior to labeling.
Remove Luer lock plugs and remove approximately 80 μL of ethanol PBS solution from channel outlet. Subsequently pipette 120 μL of wash buffer 1 into inlet followed by removal from outlet (repeat twice).
Remove 80 μL of wash buffer 1 solution from the channel outlet and pipette 120 μL of FISH Buffer (containing all probes) into the channel inlet. Subsequently, remove an additional 60 μL of wash buffer 1 from the channel outlet and add 60 μL of FISH Buffer to the channel inlet. Seal the channel with a Luer lock plug and place in a hybrid oven at 46°C overnight.
Upon completion, remove the FISH buffer from the channel outlet, then add 80 μL of wash buffer 1 to the channel inlet. Follow by removing an additional 60 μL of wash buffer 1 from the channel outlet and add another 60 μL of wash buffer 1 to the channel inlet. Let it incubate at 48°C for 15 minutes.
After incubation, remove wash buffer 1 from the channel outlet and add 80 μL of wash buffer 2 to the channel inlet. Let it incubate at 48°C for an additional 10 minutes.
Dehydrate by pipetting 2x 120 μL each of 50% EtOH, 70% EtOH, and 100% EtOH. Remove all remaining EtOH in flow channel and allow flow channel to dry completely.
Mount in SlowFade Gold Antifade Mountant (pipet approximately 100 μL into channel).
Multispectral Image Acquisition and Linear Unmixing
-
Use microscope ocular to find a section of biofilm containing all label types.
This may require searching via live scan modes for fluorescent labels that are not visible to the eye under epifluorescence. This step is required in order to select microscope settings to optimally excite all fluorophores present in the sample without saturating pixels.
Begin setting the image acquisition parameters in scan mode. Optimize acquisition settings starting with the shortest wavelength laser (488 nm). This excitation wavelength will maximally excite AF488, AF514. Set the laser power such that the dynamic range in the image is maximized but no pixels are saturated in any spectral channel. Leaving on the 488 nm laser, repeat the process with the 561 nm laser to excite AF555, AF594, RRX. Leaving both 488 nm and 561 nm laser on, repeat again with the 633 nm laser to excite AF647 and AF660.
-
Determine the number of channels to record. When using a 32-element detector, the maximum number of channels is 32.
Not all channels may be necessary for the specific experiment. For example, the shortest excitation wavelength in this protocol is 488 nm, so it is not necessary to collect wavelengths below 488 nm.
Acquire spectral images of singly labeled samples by labelling E.coli samples with PacBlue, AF488, AF514, AF555, RRX, AF594, AF647 and AF660. The singly labeled samples will be acquired with identical settings as the multi-labeled image.
Extract reference spectra from the singly labelled E. coli cell images for each of the seven fluorophores used in the experiment. Reference spectra will be used as inputs for a linear unmixing (LU) algorithm.
Apply LU to the multi-labeled biofilm sample.
After unmixing determine the quality of the image visually and determine if settings need to be adjusted. If settings are adjusted, then repeat steps 5 and 6.
Reagents and Solutions
FISH Buffer
0.9 M NaCl (use 5 M NaCl stock solution)
0.02 M Tris pH 7.5 (use 1 M Tris stock solution)
0.01% sodium dodecyl sulfate (SDS)
20% HiDi formamide (Thermofisher Cat# 4440753)
Milli-Q ultrapure water
FISH Probes (See Table 1 for probe sequences and fluorophore characteristics):
Table 1:
Salient characteristics of fluorescence in situ hybridization probes for 7 genera of bacteria abundant in dental plaque and for all bacteria as used in the protocol here.
| Fluorophore | Abbreviation | Peak Excitation λ (nm) |
Peak Emission λ (nm) |
Probe Name |
Target | Sequence | Reference |
|---|---|---|---|---|---|---|---|
| Pacific Blue | PacBlue | 410 | 455 | EUB338 | All bacteria | GCTGCCTCCCGTAGGAGT | Amann 1990 |
| Alexafluor 488 | AF488 | 495 | 519 | ACT476 | Actinomyces | ATCCAGCTACCGTCAACC | Gmur 2004 |
| Alexafluor 514 | AF514 | 517 | 542 | VEI488 | Veillonella | CCGTGGCTTTCTATTCCG | Chalmers 2008 |
| Alexafluor 555 | AF555 | 555 | 565 | FUS714 | Fusobacterium | GGCTTCCCCATCGGCATT | Valm 2011 |
| Rhodamine Red-X | RRX | 560 | 580 | PAS111 | Pasteurellaceae | TCCCAAGCATTACTCACC | Valm 2011 |
| Alexafluor 594 | AF594 | 590 | 617 | STR405 | Streptococcus | TAGCCGTCCCTTTCTGGT | Paster 1998 |
| Alexafluor 647 | AF647 | 650 | 665 | COR633 | Corynebacterium | AGTTATGCCCGTATCGCCTG | Valm 2011 |
| Alexafluor 660 | AF660 | 663 | 690 | LEP568 | Leptotrichia | GCCTAGATGCCCTTTATG | Valm 2011 |
Each probe should be added to achieve a final concentration of 2 μM in the FISH Buffer.
AF488-ACT476
AF514-VEI488
AF555-FUS714
RRX-PAS111
AF594-STR405
AF647-COR633
AF660-LEP568
PacBlue-EUB338
Mineral Salt Buffer
Sodium phosphate dibasic (Na2HPO4): 1.15 g/L
Sodium chloride (NaCl): 3.00 g/L
Potassium chloride (KCl): 0.20 g/L
Potassium phosphate monobasic (KH2PO4): 0.20 g/L
Magnesium sulfate heptahydrate (MgSO4 · 7H2O): 0.10 g/L
Wash Buffer 1
0.9 M NaCl (use 5 M NaCl stock solution)
0.02 M Tris pH 7.5 (use 1 M Tris stock solution)
0.01% sodium dodecyl sulfate (SDS)
20% HiDi formamide (Thermofisher Cat. No. 4440753)
Milli-Q ultrapure water
Wash Buffer 2
0.9 M NaCl (use 5 M NaCl stock solution)
0.02 M Tris pH 7.5 (use 1 M Tris stock solution)
0.01% sodium dodecyl sulfate (SDS)
Milli-Q ultrapure water
COMMENTARY
Background Information
The human oral microbiome is a diverse community of fungi, viruses and over 700 known species of bacteria, a subset of which live as members of the dental plaque biofilm on tooth surfaces (Abusleme et al., 2021; Dewhirst et al., 2010). Compositional changes in dental plaque microbiome communities in the transition from health to disease, termed dysbiosis have been well-documented; however, changes in the spatial structure of these communities during disease progression have not been well studied (Lamont et al., 2018). Mapping the systems level structure of dental plaque biofilms is critical for understanding the spatio-temporal and functional changes that occur in oral microbial communities during the transition from health to periodontal disease and caries.
In vitro oral microcosm communities, seeded with mixed communities of human dental plaque have proven to be an essential tool for studying the taxonomic composition of dental plaque communities and the changes in community composition in response to specific perturbations. Compositional analyses of these models have shown them to support the growth of many dozens of organisms in co-culture(Cieplik et al., 2018; Edlund et al., 2013; Mostajo et al., 2017). However, these models are usually grown in media that may not be representative of human saliva or gingivocrevicular fluid and may be grown on optically opaque surfaces, e.g., hydroxyapatite or other materials, e.g., plastic that are incompatible with high resolution, multispectral fluorescence imaging (Valm et al., 2011).
Our method introduces a systematic approach for investigating the effects of chemical interventions, varying environmental conditions, and nutrient sources, specifically on the micron-scale spatial structure of oral microcosms because the substrate is a microscope coverslip. Alternative approaches that utilize hydroxyapatite discs as a substrate may better recapitulate the tooth surface on which dental plaque grows; but this substrate is difficult to image since it is opaque (Cieplik et al., 2018; Janus et al., 2016). Synthetic communities with mixtures of pure isolates provide controlled environments and spatial insights (Liu et al., 2018). However, they do not capture the complete spectrum of taxa typically present in genuine oral samples. The main disadvantage of our technique is that the glass substrate may not fully recapitulate the mineralized tooth substrate on which natural dental plaque communities grow.
Critical Parameters
Several parameters affect the ability to map the spatial structure of oral microcosms cultured via the methods described in this protocol. When assembling and priming the biofilm flow system, it is critical to minimize the introduction and formation of bubbles in the lines. Bubble formation may be unavoidable during, e.g., cellular respiration, therefore it is critical to orient the flow cell at an angle to sequester bubbles in one area of the flow cell. For FISH probe choice, the user may rely on known abundance and prevalence information in human dental plaque; however, the high inter-individual variability that has been reported in this community may justify DNA sequencing of replicate biofilms to identify organisms of interest for targeting with FISH probes.
For robust and accurate linear unmixing using reference standards as described here, it is imperative that the biofilm images be acquired on the microscope using the same image acquisition settings as the reference images (Zimmermann, 2005). In practice, this means that the acquisition settings should first be set using the biofilm sample, on a region of the heterogeneous community where all fluorescent labels are present. The dynamic range of the multispectral image should be maximized for each excitation laser used. Once these settings are optimized, the same settings should be used for acquiring spectral reference images from each E. coli sample labeled with a unique fluorophore to extract spectra for LU. In practice, reference images may be higher intensity than the biofilm image. Saturation in pixels must be avoided as it is incompatible with linear unmixing. If reference images are much brighter than the biofilm images and saturation is present in the recorded image, the excitation energy must be decreased in such a way that all spectral channels are decreased proportionately. This may be achieved through: 1. decreasing the pixel dwell time or 2. decreasing the laser power proportionately for each laser, but the user should be aware that laser power output is linear over a finite range of power settings on the instrument, and is expected to be non-linear towards the minimal and maximal power settings.
Troubleshooting
During the execution of the 'Dental Plaque on a Chip In Vitro Culture Model', researchers might encounter various challenges. To assist in addressing these potential issues and ensuring a successful experiment, we've outlined common problems, their potential causes, and recommended solutions in Table 2.
Table 2.
Troubleshooting Guide for Dental Plaque on a Chip In Vitro Culture Model
| Problem | Possible Cause | Solution |
|---|---|---|
| Bubbles disrupt biofilm | Air introduced through injection port, cellular respiration. | Pay careful attention to inoculation and stop injection before air is introduced. Place channel slide on an angle so that flow occurs up a slope allowing bubbles to float up away through flow channel. |
| Biofilm overgrown | Media too concentrated, culture time too long, or cell density too high. | Dilute media systematically in di H2O, adjust culture time, or dilute inoculation cell density. |
| Biofilm lacks diversity | Dental plaque obtained from volunteer contains low level of viable cells, culture time too short, or media is too dilute. | Dental plaque viability can be verified by live/dead bacterial staining and multispectral labeling.
|
| A labeled taxon is not detected in sample | Oral biofilms are heterogenous in spatial structure and therefore certain taxa may not be present in everywhere in the sample | Image across a larger field of view. Consider acquiring tile scan images to increase the statistical probability that all taxa will be captured in the imaged volume. |
Understanding Results
Biofilms harvested after 24-48 hours of growth are expected to have taxonomic diversity similar to the initial inoculum, with some taxa overrepresented and others under-represented or completely absent (Fig. 4). The culture conditions described here are designed to recapitulate the in vivo formation of dental plaque on teeth; however, some components are missing from this model, e.g., the host immune system which is known to influence oral biofilm composition(Williams et al., 2021). Fluorescence intensity in the recorded image may be low for some taxa if those organisms were not metabolically active at the time of biofilm fixation, but in general, the rich media supplied here and the availability of unoccupied niches on the slide surface for biofilm cell attachment should promote the growth of diverse and metabolically active microbes. After fixation, biofilms are stable and can be stored for 6 months or more at 4 C in 50% ethyl alcohol, though care should be taken to ensure that biofilms do not dry during storage. Inter-individual heterogeneity in dental oral microbiome composition, especially at the strain level will result in heterogeneous in vitro biofilms. Dental plaque samples from multiple donors may be pooled before inoculation to reduce variability from biological replicates.
Figure 4:
Anticipated result after Linear Unmixing (LU). (A) Micrographs of oral biofilms cultured in dental plaque on a chip model, labeled for Fusobacterium, Leptotrichia, Actinomyces, Veillonella, Streptococcus, Corynebacterium, Pasteurellaceae, and all bacteria with EUB338 general bacterial probe. Linear unmixing allows abundance estimation of each fluorophore at every pixel. Scale bars, 20 μm. (B) Zoom field of view of the region highlighted with dashed lines in (A). Scale bars 10 μm.
Time Considerations
Prior to in vitro flow culture setup
Filter sterilization of mucin containing medium is slow and therefore this step should be done before running the in vitro flow cell experiment. In this protocol we use a Nalgene Rapid Flow bottle top filters with 50 mm diameter 0.2 μm PES membrane filter. Each filter can sterilize approximately 30ml of dilute GM medium [~5 mins/30ml].
On day of in vitro flow culture setup [~8 hours]
Sufficient time [~ 1 hour] should be allocated for flow system setup prior to hybridization of saliva to μ-slide channels [1 hour]; this allows for media too reach 37C during saliva hybridization step. Inoculation of donor plaque into each flow channel occurs at 0 hours, 3 hours and 6 hours [6 hours]. All culturing steps can be completed in a day.
Imaging
Acquisition of spectral z-stacks in thick biofilm sections requires additional time compared to nonspectral imaging and time must also be allocated for LU of spectral images. Approximately 30 minutes are required for the acquisition of each z-stack assuming similar settings to that used for the image in figure 4 (Scan Mode: LineSequential, Pixel Time: 1.24 μs, Scan Direction: Unidirectional, Averaging: 2, Lamda mode: 32 channels, image size: 1584 by 1584 pixels, pixel scaling: 0.09 μm x 0.09 μm x 0.320 μm, Z-stack: 127 Slices).
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grant R01DE030927.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DATA AVAILABILITY STATEMENT
Data will be made available upon request to the authors.
LITERATURE CITED
- Abusleme L, Hoare A, Hong B & Diaz PI (2021). Microbial signatures of health, gingivitis, and periodontitis. Periodontology 2000, 86(1), 57–78. 10.1111/prd.12362 [DOI] [PubMed] [Google Scholar]
- Cieplik F, Zaura E, Brandt BW, Buijs MJ, Buchalla W, Crielaard W, Laine ML, Deng DM & Exterkate RAM (2018). Microcosm biofilms cultured from different oral niches in periodontitis patients. Journal of Oral Microbiology, 11(1), 1551596. 10.1080/20022727.2018.1551596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Valm AM & Lippincott-Schwartz J (2018). Multispectral Live-Cell Imaging. Current Protocols in Cell Biology, 79(1), e46. 10.1002/cpcb.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner ACR, Yu W-H, Lakshmanan A & Wade WG (2010). The Human Oral Microbiome. Journal of Bacteriology, 192(19), 5002–5017. 10.1128/jb.00542-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund A, Yang Y, Hall AP, Guo L, Lux R, He X, Nelson KE, Nealson KH, Yooseph S, Shi W & McLean JS (2013). An in vitrobiofilm model system maintaining a highly reproducible species and metabolic diversity approaching that of the human oral microbiome. Microbiome, 1(1), 25. 10.1186/2049-2618-1-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammon ST, Leevy WM, Gross S, Gokel GW & Piwnica-Worms D (2006). Spectral Unmixing of Multicolored Bioluminescence Emitted from Heterogeneous Biological Sources. Analytical Chemistry, 78(5), 1520–1527. 10.1021/ac051999h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janus MM, Crielaard W, Zaura E, Keijser BJ, Brandt BW & Krom BP (2016). A novel compound to maintain a healthy oral plaque ecology in vitro. Journal of Oral Microbiology, 8(1), 32513. 10.3402/jom.v8.32513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont RJ, Koo H & Hajishengallis G (2018). The oral microbiota: dynamic communities and host interactions. Nature Reviews Microbiology, 16(12), 745–759. 10.1038/s41579-018-0089-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Russel J, Burmølle M, Sørensen SJ & Madsen JS (2018). Micro-scale intermixing: a requisite for stable and synergistic co-establishment in a four-species biofilm. The ISME Journal, 12(8), 1940–1951. 10.1038/s41396-018-0112-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostajo MFY, Exterkate RAM, Buijs MJ, Beertsen W, Weijden G. A. van der, Zaura E & Crielaard W (2017). A reproducible microcosm biofilm model of subgingival microbial communities. Journal of Periodontal Research, 52(6), 1021–1031. 10.1111/jre.12473 [DOI] [PubMed] [Google Scholar]
- Valm AM, Welch JLM, Rieken CW, Hasegawa Y, Sogin ML, Oldenbourg R, Dewhirst FE & Borisy GG (2011). Systems-level analysis of microbial community organization through combinatorial labeling and spectral imaging. Proceedings of the National Academy of Sciences, 108(10), 4152–4157. 10.1073/pnas.1101134108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Lemus A, Henneberry C, Feng Y & Valm AM (2022). Biological and Technological Tools to Probe Systems Level Structure of Human Oral Biofilms. Microscopy and Microanalysis, 28(S1), 1546–1547. 10.1017/s1431927622006213 [DOI] [Google Scholar]
- Williams DW, Greenwell-Wild T, Brenchley L, Dutzan N, Overmiller A, Sawaya AP, Webb S, Martin D, Core NG and C. B., Hajishengallis G, Divaris K, Morasso M, Haniffa M & Moutsopoulos NM (2021). Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell, 184(15), 4090–4104.e15. 10.1016/j.cell.2021.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann T (2005). Spectral Imaging and Linear Unmixing in Light Microscopy. Advances in Biochemical Engineering/Biotechnology, 245–265. 10.1007/b102216 [DOI] [PubMed] [Google Scholar]
- Zimmermann T, Marrison J, Hogg K & O’Toole P (2013). Confocal Microscopy, Methods and Protocols. Methods in Molecular Biology, 1075, 129–148. 10.1007/978-1-60761-847-8_5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon request to the authors.