Abstract
Background
Context fear memory can be reliably reduced by subsequent pairings of that context with a weaker shock. This procedure shares similarities with extinction learning: both involve extended time in the conditioning chamber following training and reduce context-elicited fear. Unlike extinction, this weak-shock exposure has been hypothesized to engage reconsolidation-like processes that weaken the original memory.
Methods
We directly compared the weak-shock procedure with extinction using male and female Long Evans rats.
Results
Both repeated weak-shock exposure and extinction resulted in decreased context freezing relative to animals that received context fear conditioning but no subsequent context exposure. Conditioning with the weak shock was not enough to form a persistent context-shock association on its own, suggesting that the weak-shock procedure does not create a new memory. Weak-shock exposure in a new context can still reduce freezing elicited by the training context, suggesting that it reduces responding through a different process than extinction, which does not transcend context. Finally, reduced fear behavior produced through both extinction and weak-shock exposure was mirrored by reduced zif268 expression in the basolateral amygdala. However, only the weak-shock procedure resulted in changes in lysine-48 polyubiquitin tagging in the synapse of the basolateral amygdala, suggesting that this procedure produced long-lasting changes in synaptic function within the basolateral amygdala.
Conclusions
These results suggest that the weak-shock procedure does not rely on the creation of a new inhibitory memory, as in extinction, and instead may alter the original representation of the shock to reduce fear responding.
Keywords: Basolateral amygdala, Context fear conditioning, Extinction, Memory, Updating
Fear-based anxiety disorders are the most prevalent class of neuropsychiatric conditions in the United States (1), yet treatments for them consistently fail to reduce symptoms in the long term (2,3). Associative learning is at the root of these disorders (4, 5, 6), and fear and anxiety can occur because of the similarity between present circumstances and situations that were previously paired with aversive outcomes (4). Extinction-based exposure therapies reduce fear symptoms. In such therapies, patients are repeatedly presented with cues (conditional stimulus [CS]) that were previously paired with aversive events (unconditional stimulus [UCS]) (7, 8, 9).
In extinction, fear responding decreases with repeated presentations of an unreinforced CS. Extinction is the basis of exposure-based therapies (4,10, 11, 12, 13, 14, 15, 16, 17) and results in the creation of an inhibitory memory that competes with the original memory for expression (18,19). Reductions in fear regulated by the basolateral amygdala (BLA) following extinction rely on retrieval of inhibitory learning acquired during extinction. Several factors can lead to a return of extinguished responding, including removal from the extinction context, UCS re-exposure, or passage of time (20,21). This suggests that original learning remains intact throughout extinction and will readily return if circumstances change. Relapse phenomena are the most significant hurdle to treatment of neuropsychiatric diseases associated with maladaptive fear responding, including specific phobias and posttraumatic stress disorder (20,22).
Behavior can also be reduced by targeting the original memory, taking advantage of a brief time period following memory retrieval during which memory becomes sensitive to disruption known as reconsolidation. Amnesiac events (23,24) or pharmacological assaults (25) that occur during reconsolidation will weaken the original memory. Recent applications have used behavioral manipulations during reconsolidation to bidirectionally change the content of the memory to be either more (26) or less (27, 28, 29, 30) aversive. These effects are dependent on calcium-permeable AMPA receptors within the amygdala (29), suggesting targeting the original memory instead of creating a new memory.
Following conditioning, the CS controls behavior through its ability to activate a representation of the UCS (31). This has been confirmed through UCS deflation studies, in which a CS is paired with food, leading to conditional responding to that CS alone. Later, the food outcome is paired with illness. This decreases CS-elicited responding relative to groups with no UCS-illness pairings (32). Thus, animals flexibly gauge responding to the CS based on the current value of the UCS that the CS predicted.
Activity and plasticity in the BLA is critical for formation, storage, and retrieval of fear memories (33, 34, 35, 36). During retrieval, memory is made labile through degradation of synaptic connections (37) mediated by the ubiquitin-proteasome system. Lysine-48 (K48) polyubiquitin tags specific proteins for degradation. Changes in synaptic K48 are crucial for both memory retrieval and destabilization in the amygdala and hippocampus (38, 39, 40, 41). The dorsal hippocampus (DH) encodes contextual information and interacts with the BLA during fear memory formation and retrieval (42,43). During retrieval, hippocampal activity gates the ability to modify fear memory in the amygdala (44,45).
Memory can be bidirectionally modulated by presenting either few or many weak versions of the UCS. Animals exposed to a brief updating procedure (two 0.3-mA shocks) following contextual fear conditioning (with five 1.0-mA shocks) showed increases in fear responding (26). Somewhat paradoxically, ten 0.3-mA shocks reduced fear to the conditioning context. Similar behavioral results were obtained using an auditory fear conditioning procedure to reduce fear to a discrete CS (28), suggesting that this method is robust across behavioral paradigms. Increased fear following 2 weak shocks resulted in increased cellular activity and synaptic destabilization within the BLA, but the molecular processes associated with behavior following 10 weak shocks are unclear.
Several possible explanations exist for the behavior-weakening effect produced by 10 weak shocks (26). First, it is possible that this 10-shock procedure could have engaged a reconsolidation-like mechanism, which would reduce fear to the context by updating the context-shock association to reflect the weak shock. Alternatively, this procedure may engage an extinction-like mechanism, which would reduce fear to the context through prolonged exposure to that context in the absence of the fear-provoking 1.0-mA shock, but lower fear would be context dependent (11,16) and especially prone to relapse. The procedure with 10 weak shocks could also have engaged a UCS deflation–like process (32) that would reduce behavioral responding by changing the UCS value. The current experiments were designed to directly compare the 10-shock deflation procedure with extinction and examine changes in retrieval-induced molecular processes within the BLA and DH.
Methods and Materials
Subjects
Subjects were age-matched female (175–199 g) and male (250–274 g) Long Evans rats from Envigo. The colony was maintained on a 12-hour light/dark cycle. Behavior occurred during the light cycle. Animals were acclimated to the colony for 7 days prior to experimentation. Animals were handled for 2 days prior to behavioral training. Male and female rats were run in separate experimental sessions.
Apparatus
Behavior occurred in Colbourn conditioning chambers, each housed in its own sound-attenuating cubicle. Each chamber consisted of plexiglass front, rear, and top walls with brushed stainless steel side walls. The floor consisted of a shock grid with 18 rods (0.4-cm diameter) spaced 1 cm apart. A fan produced continuous 65-dB noise. Chambers were lit with a white light-emitting diode houselight. Between each animal, chambers were thoroughly cleaned with water. For experiments using 2 contexts, a second set of chambers was introduced. In these chambers, no noise was played, the light-emitting diode light was red, and the chambers were cleaned with bleach between animals. White- and red-lit chambers were counterbalanced as context A and context B.
Training
Animals were assigned to one chamber and placed inside on the first day. After 2 minutes, animals received five 1-second 1.0-mA footshocks with an interstimulus interval of 1 minute. Animals were removed 2 minutes following the final footshock. In one experiment (Figure 1F), a 0.3-mA footshock was used for fear conditioning.
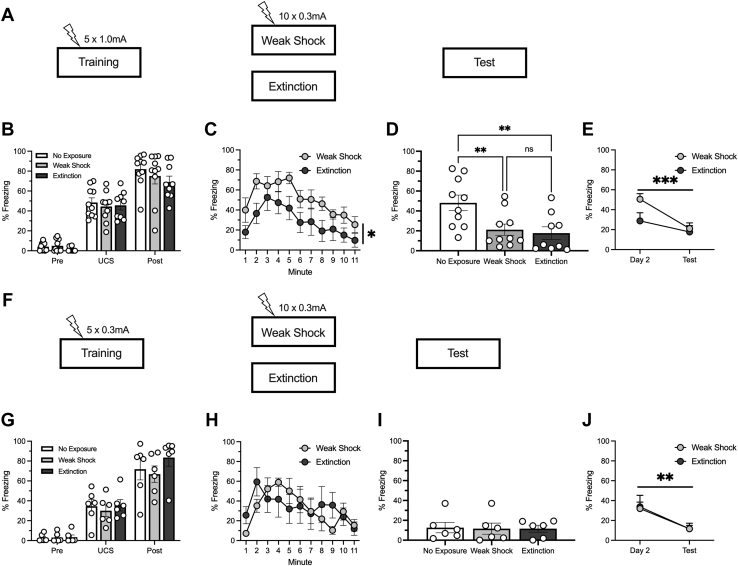
Figure 1.
Weak-shock presentations following context fear conditioning reduce freezing but are not enough to create a persistent fear memory on their own. (A) Behavioral design. All animals received 5 context-shock pairings during training. The next day, animals received either 10 weak-shock presentations (weak shock), exposure to the context alone (extinction), or remained in the home cage (no exposure). On the final day, all animals were returned to the training context for testing. Group sizes were as follows: weak shock, n = 10 (5 female, 5 male); extinction, n = 9 (4 female, 5 male); no exposure, n = 10 (5 female, 5 male). (B) All groups increased their freezing throughout training. (C) Both groups reduced their freezing throughout the day 2 session, although overall responding was higher in the weak-shock group. (D) Both weak-shock and extinction groups showed reduced freezing relative to the no-exposure control group. (E) Both weak-shock and extinction groups showed reduced freezing relative to their freezing on day 2, but this was more pronounced in the weak-shock group. (F) Behavioral design. All animals received 5 context–weak shock pairings during training. The next day, animals received either 10 weak-shock presentations (weak shock), exposure to the context alone (extinction), or remained in the home cage (no exposure). On the final day, all animals were returned to the training context for testing. Group sizes were as follows: weak shock, n = 6 (3 female, 3 male); extinction, n = 6 (3 female, 3 male); no exposure, n = 6 (3 female, 3 male). (G) All groups increased their freezing throughout acquisition. (H) Both groups reduced their freezing throughout the day 2 session. (I) All groups showed similarly low levels of freezing during the test. (J) Both weak-shock and extinction groups showed reduced freezing relative to their freezing on day 2. ∗p < .05, ∗∗p < .01, ∗∗∗p < .001. UCS, unconditional stimulus.
Day 2 (Weak Shock or Extinction)
Twenty-four hours later, the animals were split into 3 experimental conditions. Animals in the weak-shock condition were placed in the conditioning chamber and after 1 minute, ten 1-second 0.3-mA footshocks were delivered with an interstimulus interval of 1 minute. Animals were removed from the chamber 15 seconds following the final footshock. Animals in the extinction condition were placed in the chamber for an equivalent amount of time (615 seconds), but no footshocks were delivered. Animals in the no-exposure condition remained in their homecages.
Testing
The next day, animals were tested for fear elicited by the conditioning chamber for 10 minutes.
Tissue Processing
Animals from the experiment depicted in the top half of Figure 1 were sacrificed 60 minutes following the test along with a control group of rats that arrived at the same time but never received any behavioral training or testing. Animals were deeply anesthetized with isoflurane, and brains were removed and immediately flash frozen. Tissue was later sliced and dissected for immunofluorescence and Western blotting.
Immunofluorescence
Immunofluorescence proceeded similarly to previous work (46, 47, 48). The tissue was sliced in 40-μm sections and mounted onto charged slides. Slides were fixed in a 10% buffered formalin before being rehydrated in wash buffer (phosphate-buffered saline [PBS] + 0.05% Tween 20) and permeabilized (PBS + 0.3% Triton X) for 15 minutes and incubated in blocking solution (PBS + 0.7% normal goat serum). Slides were incubated in zif268/EGR-1 antibody (1:400; Cell Signaling) solution (PBS + 0.3% Triton X + 5% normal goat serum) overnight at 4 °C. The next day, tubes were placed at room temperature 2 hours before incubation in a secondary antibody solution for 2 hours. Slides were rinsed with wash buffer, a DAPI counterstain was applied, and coverslipped. Images were captured from the CA1 region of the DH and the BLA on the Leica THUNDER imager system using a 20× objective using LAS-X (Leica). Images were exported as 12-bit TIFF files and converted to a binary image via Gaussian filtering (sigmas: 6, 3) and then quantified using the Analyze Particles plugin in ImageJ (imagej.net). zif268 activity was normalized as a proportion of DAPI present in the same section. Groups showed no difference in DAPI levels in the DH (F = 1.61, p = .188) or BLA (F = 1.11, p = .347).
Synaptosomal Preparation
K48 polyubiquitin tagging within the synaptic compartment is associated with memory reconsolidation-like processes (49). Crude synaptosomal fractions were obtained as previously described (45). The amygdalae were homogenized in TEV protease buffer with 320 mM sucrose and centrifuged at 1000g for 10 minutes. The supernatant was removed and centrifuged at 10,000g for 10 minutes, and the remaining pellet was denatured in lysis buffer (all in 100 mL DDH2O; 0.605 g Tris-HCl, 0.25 g sodium deoxycholate, 0.876 g NaCl, 1 μg/mL phenylmethylsulfonyl fluoride (PMSF), 1 μg/mL leupeptin, 1 μg/mL aprotinin, 10 mL 10% SDS [sodium dodecyl sulfate]).
Western Blotting
Following synaptosomal preparation, proteins were loaded onto a 7.5% SDS-PAGE (SDS–polyacrylamide gel electrophoresis) gel and then to a membrane using a transfer apparatus (Trans-blot Turbo Transfer System; Bio-Rad). Membranes were incubated in blocking buffer (50% TBS blocking buffer [LI-COR Biosciences], 50% TBS + 0.1% Tween 20) for 1 hour and then incubated in K48 (1:500; Cell Signaling) or β-actin (1:1000; Cell Signaling) primary solutions overnight at 4 °C. Membranes were rinsed and washed 3 times for 5 minutes in wash buffer (TBS + 0.1% Tween 20) and then incubated in appropriate secondary (1:15,000; IRDye 800CW goat anti-rabbit, IRDye 680RD goat anti-mouse; LI-COR) antibody for 1 hour at room temperature. Images were captured using the Odyssey Fc near-infrared system (LI-COR). Densitometry was performed using Image Studio. K48 was first normalized to actin (in which no group differences were observed) (F = 1.14, p = .279).
All immunofluorescent and Western blot data were expressed as a percentage relative to naïve animals that arrived in the colony at the same time, were housed and maintained the same way, and were sacrificed at the same time as experimental rats (n = 9; 4 female, 5 male). These animals were never given any behavioral experience or removed from the colony prior to tissue collection.
Data Analysis
All data were analyzed with analyses of variance (ANOVAs) or t tests (alpha = 0.05) using SPSS (version 29; IBM). Planned comparisons assessed between- and within-group differences following main effects or interactions. Data are presented as group means and stratified by sex in Figures S1–S5.
Results
Weak-Shock Exposure and Extinction Reduced Context Fear Responding
We first compared the weak-shock procedure with extinction following contextual fear conditioning (design in Figure 1A). Groups were compared with a no-exposure control group that received training and testing but remained in their homecage during day 2.
Training
All animals similarly increased their freezing during training (Figure 1B). This was confirmed by a 3 (group) × 3 (time period) ANOVA that found a main effect of time period (F2,52 = 261.27, p < .001) but no main effect of group or an interaction (Fs < 1).
Day 2 (Weak Shock or Extinction)
To assess responding throughout this session (Figure 1C), a 2 (group) × 11 (minute) ANOVA was conducted. This found main effects of group (F1,17 = 5.05, p = .038) and minute (F10,170 = 9.25, p < .001) but no interaction between the two (F < 1). The extinction group froze less than the weak-shock group, but both groups decreased freezing throughout the session.
Test
A one-way ANOVA compared freezing during the test session (Figure 1D) and found an interaction (F2,26 = 6.27, p = .006). While the no-exposure group froze more than the weak-shock (p = .007) and extinction (p = .004) groups, the weak-shock and extinction groups did not differ from each other (p = .720). To examine how responding changed within the group between phase 2 and the test, a 2 (group) × 2 (session) ANOVA was conducted (Figure 1E). This found a main effect of session (F1,17 = 27.66, p < .001) and an interaction (F1,17 = 5.69, p = .029) but no main effect of group (F1, 17 = 2.35, p = .14), demonstrating that while both groups decreased responding from day 2 to testing, this decrease was larger following weak shock. Planned comparisons demonstrated that while the weak-shock group showed a significant decrease during this time (p < .001), this was only a trend in the extinction group (p = .064).
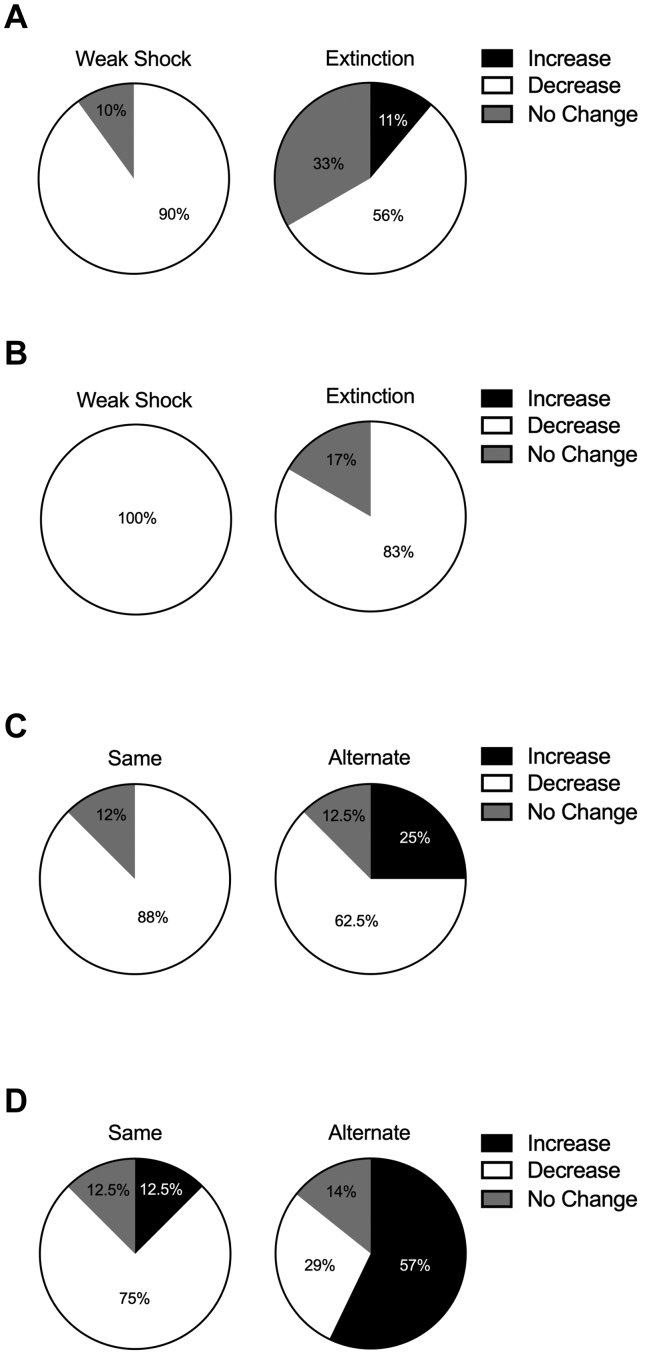
Rats were then designated as having either decreased or increased responding between these days. If animals did not change responding by more than 5% in either direction, they were designated as no change. Ninety percent of the weak-shock condition animals reduced their freezing between sessions, and 56% of the extinction group did so (Figure 3A).
Figure 3.
Within-subject changes between day 2 and testing demonstrate unique behavioral outcomes of extinction and weak shock. (A) Ninety percent of animals in the weak-shock group and 56% of animals in the extinction group showed a decrease in responding between day 2 and testing. (B) One hundred percent of animals in the weak-shock group and 83% of animals in the extinction group showed a decrease in responding between day 2 and testing. (C) Eighty-eight percent of animals that received weak shock in the same context and 62.5% of animals that received weak shock in an alternative context showed a decrease in responding between day 2 and testing. (D) Seventy-five percent of animals that received extinction in the same context showed a decrease in responding between day 2 and testing. For the first and only time in this set of experiments, a majority of animals (57%) in the alternative group showed an increase in responding between day 2 and testing.
Exposure to the Weak Shock Resulted in Unconditional Freezing to the Context Without Creating a Persistent Fear Memory
Next, we aimed to test whether the weak shock itself was enough to support a fear memory. All animals were first conditioned with the weak (0.3 mA) shock (design in Figure 1F). As before, both the weak-shock and extinction groups were compared with a group that did not receive behavioral manipulations between training and testing.
Training
Fear conditioning with the 0.3-mA shock increased freezing throughout the training session (Figure 1G), which was confirmed by a 3 (group) × 3 (time period) ANOVA that found a main effect of time period (F2,30 = 109.09, p < .001) but no effect of group or interaction (Fs < 1). This suggests that weak shock can produce unconditional freezing.
Day 2 (Weak Shock or Extinction)
A 2 (group) × 11 (minute) ANOVA assessed responding throughout this session (Figure 1H). This found a main effect of minute (F10,100 = 5.30, p < .001) and an interaction (F10,100 = 2.10, p = .031) but no effect of group (F < 1), suggesting that the weak shock sustained more consistent levels of freezing during this session.
Test
A one-way ANOVA found no differences between the groups during testing (Figure 1I). All 3 groups showed low freezing to the context, suggesting that conditioning with the 0.3-mA shock failed to create a persistent fear memory. A 2 (group) × 2 (session) ANOVA was conducted to assess behavioral decreases between day 2 and testing (Figure 1J). This found a main effect of session (F1,10 = 17.30, p = .002) but no effect of group or interaction (Fs < 1). Both weak-shock (p = .018) and extinction (p = .012) groups decreased their freezing between day 2 and testing. One hundred percent of the weak-shock group and 83% of the extinction group decreased their freezing between day 2 and testing (Figure 3B).
Effects of Weak-Shock Exposure Transferred Across Contexts
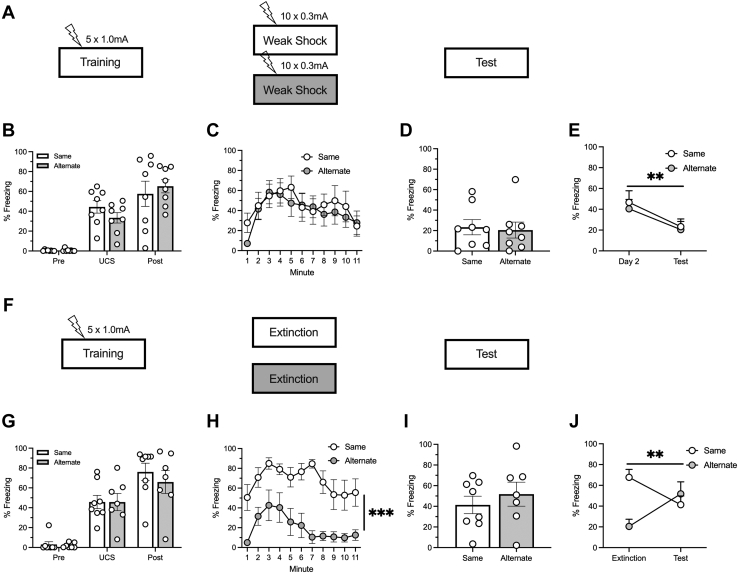
Extinction learning is characterized by its context dependency. However, contextual novelty has been shown to facilitate reconsolidation-like processes (45,50). Therefore, we compared weak shock in either the same or an alternate context (design depicted in Figure 2A).
Figure 2.
Weak-shock exposure conducted outside the training context can reduce behavior in the training context. (A) Behavioral design. All animals received 5 context-shock pairings during training. The next day, animals received 10 weak shocks in either the same context as training (same) or in a novel context (alternate). Group sizes were as follows: same, n = 8 (4 female, 4 male); alternate, n = 8 (4 female, 4 male). (B) Both groups increased their freezing throughout the training session. (C) Groups did not differ during the weak-shock phase. (D) Groups did not differ during the testing phase. (E) Both groups decreased their freezing between day 2 and testing. (F) Behavioral design. All animals received 5 context-shock pairings during training. The next day, animals received exposure to either the same context as training (same) or a novel context (alternate). Group sizes were as follows: same, n = 8 (4 female, 4 male); alternate, n = 7 (3 female, 4 female). (G) Both groups increased their freezing throughout the training session. (H) While both groups gradually decreased their freezing throughout the second day, overall, animals in the same context froze more than animals in the alternate context. (I) Groups did not differ during the testing phase. (J) While animals that received extinction in the same context decreased their responding between day 2 and the test, the opposite pattern was observed in animals that received exposure to the alternate context. ∗p < .05, ∗∗p < .01, ∗∗∗p < .001. UCS, unconditional stimulus.
Training
Animals increased their freezing throughout training (Figure 2B). This was confirmed by a 2 (group) × 3 (time period) ANOVA that found a main effect of time period (F2,28 = 60.73, p < .001) but no main effect of group or an interaction (largest F = 1.42, p = .259).
Weak Shock
A 2 (group) × 11 (minute) ANOVA assessed responding throughout the session (Figure 2C). Both groups decreased their responding throughout the session, which was indicated by a main effect of minute (F10,140 = 6.36, p < .001). There was no effect of group or interaction (Fs < 1). During the first minute of the session before any shocks were introduced, animals in the alternate context froze less than animals in the acquisition context (p = .055), suggesting that animals could discern the contexts prior to the first shock.
Test
An independent-samples t test found no difference between the groups during testing (t14 = 0.261, p = .798) (Figure 2D). A 2 (group) × 2 (session) ANOVA conducted to assess within-group differences between weak shock and testing (Figure 2E) found a main effect of session (F1,14 = 11.64, p = .004) but no effect of group or interaction (Fs < 1). Planned comparisons showed that both same (p = .021) and alternate (p = .043) groups decreased freezing over this time. Eighty-eight percent of animals in the same context reduced their freezing between sessions, and 62.5% of animals that received weak shock in the alternative context did so (Figure 3C).
Exposure to a Second Context in the Absence of the Weak Shock Increased Between-Session Freezing
Next, we wanted to confirm that exposure to the alternate context in the absence of the UCS did not have the same effect as weak-shock exposure in the alternate context (design depicted in Figure 2F), akin to an ABA renewal–like design. However, given that the alternate context should not elicit a fear response, exposure to the second context does not necessarily constitute extinction.
Training
Animals increased their freezing throughout training (Figure 2G). A 2 (group) × 3 (time period) ANOVA found a main effect of time period (F2,26 = 86.31, p < .001) but no effect of group or interaction (Fs < 1).
Extinction
A 2 (group) × 11 (minute) ANOVA assessed responding throughout the session (Figure 2H) and found main effects of both time (F10,130 = 4.91, p < .001) and group (F1,13 = 20.50, p < .001) but no interaction (F < 1). Overall responding was higher in the same context than in the alternate context, and both groups decreased freezing over time.
Test
While groups showed no differences in overall freezing during the test session (Figure 2I) (t13 = 0.739, p = .473), a 2 (group) × 2 (session) ANOVA conducted to examine within-group differences between the extinction and the test session (Figure 2J) found an interaction (F1,13 = 12.87, p = .003) and an effect of group (F1,13 = 5.71, p = .033) but no effect of session (F < 1). Planned comparisons demonstrated that the same group decreased freezing throughout this time (p = .006) and that a nonsignificant trend was observed in the opposite direction in the alternate group (p = .091). Seventy-five percent of animals that received exposure to the same context showed reduced freezing between sessions, but only 29% of the animals that had exposure to an alternate context did so. This was the only group run within this series of experiments in which a majority (57%) of the animals increased their responding between sessions (Figure 3D).
Amygdala Molecular Profiles Differed Following Behavioral Reduction Achieved Through Either Weak Shock or Extinction
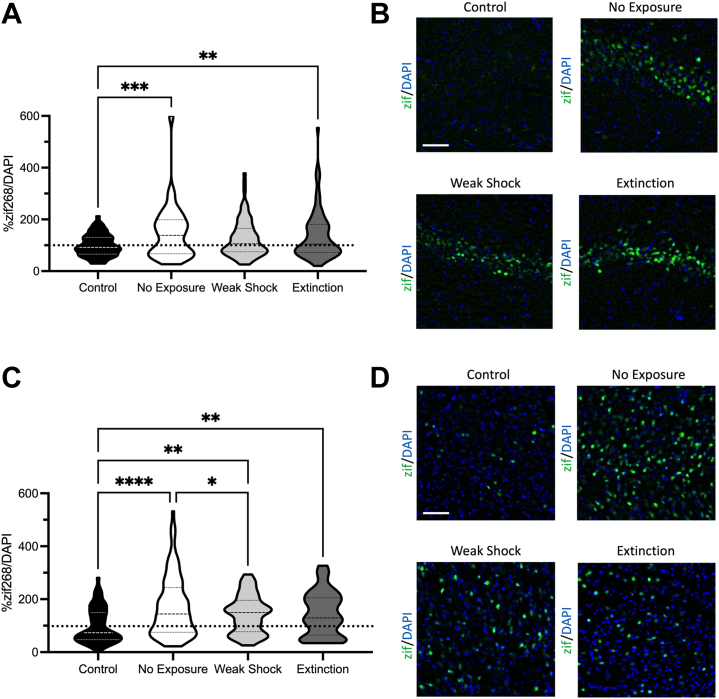
Animals were sacrificed 60 minutes following the test shown in Figure 1D, and tissue was collected for immunofluorescence and Western blotting. Activity in the DH has been associated with context learning, such that placement in a context alone is enough to drive zif268 expression in the CA1 region (51). Furthermore, immediate early gene zif268 is elevated in the BLA following fear conditioning, and increased fear is associated with higher BLA zif268 expression (36,52, 53, 54). It was previously demonstrated that with K48, a polyubiquitin chain involved in proteasomal degradation, tagging was upregulated in the synapse following memory retrieval but not memory formation (49). This polyubiquitin chain is an essential component for the synaptic changes underlying persistent fear memory modifications. Furthermore, the presentation of a few lower-intensity shocks following context fear learning that results in elevated fear increases zif268-K48 coexpression (26). Collectively, this evidence suggests that BLA activity and K48 tagging might be associated with interference during retrieval when reconsolidation-like, but not consolidation-like, effects occur.
zif268 Activity Was Generally Elevated in the DH in Groups That Received Testing
zif268 was quantified as a proportion of DAPI in that same section and then expressed as a percentage of the control group, which received no behavioral training or testing (Figure 4A). A one-way ANOVA was significant (F3,285 = 4.15, p = .007). The no-exposure (p < .001) and extinction (p = .008) groups showed increased zif268, whereas the weak-shock group only showed a trend (p = .074). This result might be attributable to the context independency of the behavioral reduction produced by the weak shock.
Figure 4.
(A) zif268 in the dorsal hippocampus expressed as a proportion of total DAPI relative to the naïve control group 60 minutes following testing. Elevated zif268 activity in the dorsal hippocampus was observed in the no-exposure and extinction groups relative to a naïve control group but not in the weak-shock group. (B) Representative images from the dorsal hippocampus in each group, with DAPI staining in blue and zif268 staining in green. (C) zif268 in the basolateral amygdala expressed as a proportion of total DAPI relative to the naïve control group 60 minutes following testing. While all 3 groups showed elevated zif268 activity in the basolateral amygdala relative to a naïve control group, both weak shock and extinction reduced zif268. (D) Representative images from the basolateral amygdala in each group, with DAPI staining in blue and zif268 staining in green. ∗p < .05, ∗∗p < .01, ∗∗∗p < .001, ∗∗∗∗p < .0001. Scale bar = 100 μM.
Both extinction and weak shock reduced zif268 expression in the BLA. Total zif268 present in each section was quantified as described above (Figure 4C; representative images in Figure 4D). A one-way ANOVA was significant (F3,296 = 7.91, p < .001). Every group showed increased zif268 activity relative to the control group (no-exposure p < .001; weak-shock p = .003; extinction p = .005). While animals in the no-exposure group had more zif268 expression than the animals in the weak-shock group (p = .031), this was only a trend relative to extinction (p = .059), suggesting that both extinction and weak-shock exposure can reduce BLA activity in addition to decreasing freezing. In both the extinction (r = 0.713, p = .031) and no-exposure (r = 0.666, p = .035) groups, DH activity was correlated with BLA activity. This was not the case in either the weak-shock (r = 0.429, p = .215) or control (r = 0.192, p = .619) group. This suggests that coordinated activity between the DH and BLA that occurs in context fear expression both before and after extinction is disrupted following the weak-shock procedure, likely due to the context independency of this effect.
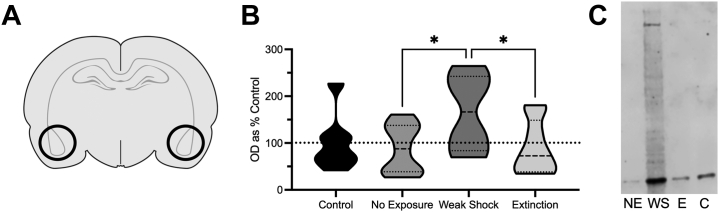
Exposure to the weak shock produced a long-lasting upregulation in BLA K48 polyubiquitin tagging. We next examined K48 within the synaptic compartment of the BLA. All experimental groups were again compared with a naïve control group that received no behavioral training or testing. A one-way ANOVA found group differences (F3,29 = 2.82, p = .057) (Figure 5B; representative lanes in Figure 5C). The weak-shock group showed elevated K48 polyubiquitination relative to both no-exposure (p = .017) and extinction (p = .020) groups and slightly elevated K48 relative to the control group (p = .051). Memory retrieval impacted synaptic function differently in this group relative to both the no-exposure group and the extinction group despite showing behavioral responding similar to the extinction group (Figure 1D).
Figure 5.
(A) Schematic of the basolateral amygdala region where tissue was collected when animals were sacrificed 60 minutes following testing. (B) Mean levels of synaptic Lysine-48 expression normalized to actin relative to a naïve control group. Lysine-48 polyubiquitin tagging was upregulated only in the weak-shock group following the test. (C) Representative blots from each group. ∗p < .05. C, control; E, extinction; NE, no exposure; WS, weak shock.
Discussion
We found that both weak shock and extinction reduced freezing relative to a group that received fear conditioning with no behavioral manipulation between training and testing. We then found that while a weak shock resulted in a small increase in contextual freezing the day following training, this memory was not persistent as evidenced by low levels of freezing in the no-exposure group during testing. Finally, we found that weak-shock exposure in a novel context reduced responding to the training context. While zif268 was generally elevated in all experimental groups relative to naïve controls in both the DH and BLA, behavior reduced through either extinction or weak-shock exposure corresponded with reduced zif268 expression in the BLA, suggesting that the amygdala was the key site of plasticity related to behavioral change. We found increased synaptic K48 polyubiquitin tagging only in the weak shock group in the BLA, demonstrating that this procedure produced long-term changes within the synapse of the BLA to promote behavior change.
When rats received the weak-shock exposure in the same or alternate context as training, we found that while animals initially discriminated between the 2 contexts, presentation of the weak shock led to freezing similar to that seen in the group that received the weak shock in the original context. This is particularly interesting because extinction, by contrast, is constrained to the extinction environment. This context dependency of extinction learning suggests that extinction results in the formation of a new inhibitory memory. This may be partially explained by the pattern of results examining cellular activity in the DH. zif268 expression was elevated in all but the weak-shock group. Together, these results suggest that the weak-shock effect does not rely entirely on new inhibitory memory like that created in extinction and instead acts on the original representation of the UCS to reduce fear responding. However, we should note that the procedure here did not directly compare UCS deflation in an alternative context with simple exposure to that context. Future work directly comparing UCS deflation to extinction in an alternative context will be especially fruitful for understanding how each procedure affects behavioral relapse.
These effects share similarities with previous work (55) in which a light predicted a loud noise, resulting in fear of the light. Following this conditioning, animals received presentations of the loud noise alone. Following this UCS habituation, animals responded less to the light relative to animals that received no habituation. However, that experiment used the same stimulus in both the training and the habituation phase. It is unlikely that using the same intensity of UCS on day 2 in our experiments would have reduced freezing behavior, and instead it would likely have resulted in enhanced fear conditioning given that we observed strong contextual fear conditioning with just 5 context-strong shock pairings. Instead, the theoretical account that can explain the current findings most completely is a UCS deflation account that suggests that rats modulate their responding to the context based on the updated value of the UCS (32). Under this account, the rats flexibly gate their responding to the context to represent the less aversive 0.3-mA shock that they experienced following fear conditioning with the strong shock.
This work is the first to directly test how reducing behaviors through presentations of a less intense UCS directly compares with behavioral reduction achieved through extinction. Future work should investigate how weak-shock exposure affects relapse effects, including spontaneous recovery and reinstatement. While the current experiments show that context fear conditioning can be reduced with weak shocks in a novel context, it has yet to be determined whether this same process will work with cued-fear conditioning. While a similar procedure was previously employed (28), this involved several short reactivations, which likely depend on reconsolidation-like mechanisms—at least at first (26). Interestingly, several short reactivations with a weak UCS functioned to reduce relapse, including renewal and spontaneous recovery (28). Systematic work directly comparing the weak-shock UCS deflation with extinction as it pertains to relapse effects will therefore need to be conducted. The current results and others (28) suggest that this procedure may be a promising avenue to mitigate relapse.
Acknowledgments and Disclosures
This work was supported by the Purdue Department of Psychological Sciences and the Purdue Institute for Integrative Neuroscience, as well as the Rosalind Franklin University of Medicine and Science Discipline of Physiology and Biophysics.
GRB and ST designed the experiments. GRB, EMH, PKR, NCF, and ST collected the data. ST analyzed the data. GRB and ST wrote the manuscript with input from NCF.
We thank the Purdue University Department of Psychological Sciences animal care staff for excellent animal care and husbandry.
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2023.01.001.
Supplementary Material
References
- 1.Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Craske M.G., Mystkowski J.L. In: Fear and Learning: From Basic Processes to Clinical Implications. Craske M.G., Hermans D., Vansteenwegen D., editors. American Psychological Association; Washington, DC: 2006. Exposure therapy and extinction: Clinical studies; pp. 217–233. [Google Scholar]
- 3.Lipp O.V., Ryan K.M., Luck C.C., Craske M.G., Waters A.M. Presentation of unpaired unconditional stimuli during extinction reduces renewal of conditional fear and slows re-acquisition. Psychophysiology. 2021;58 doi: 10.1111/psyp.13899. [DOI] [PubMed] [Google Scholar]
- 4.Bouton M.E., Mineka S., Barlow D.H. A modern learning theory perspective on the etiology of panic disorder. Psychol Rev. 2001;108:4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- 5.Jasnow A.M., Cullen P.K., Riccio D.C. Remembering another aspect of forgetting. Front Psychol. 2012;3:175. doi: 10.3389/fpsyg.2012.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maren S., Phan K.L., Liberzon I. The contextual brain: Implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craske M.G., Kircanski K., Zelikowsky M., Mystkowski J., Chowdhury N., Baker A. Optimizing inhibitory learning during exposure therapy. Behav Res Ther. 2008;46:5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Craske M.G., Treanor M., Zbozinek T.D., Vervliet B. Optimizing exposure therapy with an inhibitory retrieval approach and the OptEx Nexus. Behav Res Ther. 2022;152 doi: 10.1016/j.brat.2022.104069. [DOI] [PubMed] [Google Scholar]
- 9.Siegel P., Cohen B., Warren R. Nothing to fear but fear itself: A mechanistic test of unconscious exposure. Biol Psychiatry. 2022;91:294–302. doi: 10.1016/j.biopsych.2021.08.022. [DOI] [PubMed] [Google Scholar]
- 10.Adkins J.M., Lynch J., Gray M., Jasnow A.M. Presynaptic GABAB receptor inhibition sex dependently enhances fear extinction and attenuates fear renewal. Psychopharmacology. 2021;238:2059–2071. doi: 10.1007/s00213-021-05831-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouton M.E., Bolles R.C. Contextual control of the extinction of conditioned fear. Learn Motiv. 1979;10:445–466. [Google Scholar]
- 12.Corcoran K.A., Maren S. Factors regulating the effects of hippocampal inactivation on renewal of conditional fear after extinction. Learn Mem. 2004;11:598–603. doi: 10.1101/lm.78704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quirk G.J. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem. 2002;9:402–407. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamai N., Nakajima S. Renewal of formerly conditioned fear in rats after extensive extinction training. Int J Comp Psychol. 2000;13:137–147. [Google Scholar]
- 15.Zelikowsky M., Pham D.L., Fanselow M.S. Temporal factors control hippocampal contributions to fear renewal after extinction. Hippocampus. 2012;22:1096–1106. doi: 10.1002/hipo.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trask S., Thrailkill E.A., Bouton M.E. Occasion setting, inhibition, and the contextual control of extinction in Pavlovian and instrumental (operant) learning. Behav Processes. 2017;137:64–72. doi: 10.1016/j.beproc.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vervliet B., Baeyens F., Van den Bergh O., Hermans D. Extinction, generalization, and return of fear: A critical review of renewal research in humans. Biol Psychol. 2013;92:51–58. doi: 10.1016/j.biopsycho.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Drew M.R., Brockway E.T. Regulation of fear extinction and relapse by hippocampal engrams. Neuropsychopharmacology. 2020;45:228–229. doi: 10.1038/s41386-019-0481-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacagnina A.F., Brockway E.T., Crovetti C.R., Shue F., McCarty M.J., Sattler K.P., et al. Distinct hippocampal engrams control extinction and relapse of fear memory. Nat Neurosci. 2019;22:753–761. doi: 10.1038/s41593-019-0361-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouton M.E. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 21.Goode T.D., Maren S. Animal models of fear relapse. ILAR J. 2014;55:246–258. doi: 10.1093/ilar/ilu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maren S., Holmes A. Stress and fear extinction. Neuropsychopharmacology. 2016;41:58–79. doi: 10.1038/npp.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mactutus C.F., Riccio D.C., Ferek J.M. Retrograde amnesia for old (reactivated) memory: Some anomalous characteristics. Science. 1979;204:1319–1320. doi: 10.1126/science.572083. [DOI] [PubMed] [Google Scholar]
- 24.Misanin J.R., Miller R.R., Lewis D.J. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- 25.Nader K., Schafe G.E., Le Doux J.E. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara N.C., Jarome T.J., Cullen P.K., Orsi S.A., Kwapis J.L., Trask S., et al. GluR2 endocytosis-dependent protein degradation in the amygdala mediates memory updating. Sci Rep. 2019;9:5180. doi: 10.1038/s41598-019-41526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monfils M.H., Cowansage K.K., Klann E., LeDoux J.E. Extinction-reconsolidation boundaries: Key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Popik B., Amorim F.E., Amaral O.B., De Oliveira Alvares L.D.O. Shifting from fear to safety through deconditioning-update. eLife. 2020;9 doi: 10.7554/eLife.51207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clem R.L., Huganir R.L. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiller D., Monfils M.H., Raio C.M., Johnson D.C., LeDoux J.E., Phelps E.A. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickens C.L., Holland P.C. Conditioning and cognition. Neurosci Biobehav Rev. 2004;28:651–661. doi: 10.1016/j.neubiorev.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Holland P.C. Event representation in Pavlovian conditioning: Image and action. Cognition. 1990;37:105–131. doi: 10.1016/0010-0277(90)90020-k. [DOI] [PubMed] [Google Scholar]
- 33.Helmstetter F.J., Parsons R.G., Gafford G.M. Macromolecular synthesis, distributed synaptic plasticity, and fear conditioning. Neurobiol Learn Mem. 2008;89:324–337. doi: 10.1016/j.nlm.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fanselow M.S., LeDoux J.E. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 35.Besnard A., Caboche J., Laroche S. Recall and reconsolidation of contextual fear memory: Differential control by ERK and Zif268 expression dosage. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall J., Thomas K.L., Everitt B.J. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: Selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci. 2001;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J.L. Memory reconsolidation mediates the updating of hippocampal memory content. Front Behav Neurosci. 2010;4:168. doi: 10.3389/fnbeh.2010.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jarome T.J., Werner C.T., Kwapis J.L., Helmstetter F.J. Activity dependent protein degradation is critical for the formation and stability of fear memory in the amygdala. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jarome T.J., Helmstetter F.J. The ubiquitin–proteasome system as a critical regulator of synaptic plasticity and long-term memory formation. Neurobiol Learn Mem. 2013;105:107–116. doi: 10.1016/j.nlm.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S.H., Choi J.H., Lee N., Lee H.R., Kim J.I., Yu N.K., et al. Synaptic protein degradation underlies destabilization of retrieved fear memory. Science. 2008;319:1253–1256. doi: 10.1126/science.1150541. [DOI] [PubMed] [Google Scholar]
- 41.Hegde A.N. The ubiquitin-proteasome pathway and synaptic plasticity. Learn Mem. 2010;17:314–327. doi: 10.1101/lm.1504010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernier B.E., Lacagnina A.F., Ayoub A., Shue F., Zemelman B.V., Krasne F.B., Drew M.R. Dentate gyrus contributes to retrieval as well as encoding: Evidence from context fear conditioning, recall, and extinction. J Neurosci. 2017;37:6359–6371. doi: 10.1523/JNEUROSCI.3029-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Couto-Pereira N.S., Lampert C., Vieira A.D.S., Lazzaretti C., Kincheski G.C., Espejo P.J., et al. Resilience and vulnerability to trauma: Early life interventions modulate aversive memory reconsolidation in the dorsal hippocampus. Front Mol Neurosci. 2019;12:134. doi: 10.3389/fnmol.2019.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huff N.C., Frank M., Wright-Hardesty K., Sprunger D., Matus-Amat P., Higgins E., Rudy J.W. Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. J Neurosci. 2006;26:1616–1623. doi: 10.1523/JNEUROSCI.4964-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrara N.C., Trask S., Pullins S.E., Helmstetter F.J. The dorsal hippocampus mediates synaptic destabilization and memory lability in the amygdala in the absence of contextual novelty. Neurobiol Learn Mem. 2019;166 doi: 10.1016/j.nlm.2019.107089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trask S., Dulka B.N., Helmstetter F.J. Age-related memory impairment is associated with increased zif268 protein accumulation and decreased Rpt6 phosphorylation. Int J Mol Sci. 2020;21:5352. doi: 10.3390/ijms21155352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trask S., Pullins S.E., Ferrara N.C., Helmstetter F.J. The anterior retrosplenial cortex encodes event-related information and the posterior retrosplenial cortex encodes context-related information during memory formation. Neuropsychopharmacology. 2021;46:1386–1392. doi: 10.1038/s41386-021-00959-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trask S., Helmstetter F.J. Unique roles for the anterior and posterior retrosplenial cortices in encoding and retrieval of memory for context. Cereb Cortex. 2022;32:3602–3610. doi: 10.1093/cercor/bhab436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orsi S.A., Devulapalli R.K., Nelsen J.L., McFadden T., Surineni R., Jarome T.J. Distinct subcellular changes in proteasome activity and linkage-specific protein polyubiquitination in the amygdala during the consolidation and reconsolidation of a fear memory. Neurobiol Learn Mem. 2019;157:1–11. doi: 10.1016/j.nlm.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 50.Jarome T.J., Ferrara N.C., Kwapis J.L., Helmstetter F.J. Contextual information drives the reconsolidation-dependent updating of retrieved fear memories. Neuropsychopharmacology. 2015;40:3044–3052. doi: 10.1038/npp.2015.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jablonski S.A., Robinson-Drummer P.A., Schreiber W.B., Asok A., Rosen J.B., Stanton M.E. Impairment of the context preexposure facilitation effect in juvenile rats by neonatal alcohol exposure is associated with decreased Egr-1 mRNA expression in the prefrontal cortex. Behav Neurosci. 2018;132:497–511. doi: 10.1037/bne0000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Espejo P.J., Ortiz V., Martijena I.D., Molina V.A. Stress-induced resistance to the fear memory labilization/reconsolidation process. Involvement of the basolateral amygdala complex. Neuropharmacology. 2016;109:349–356. doi: 10.1016/j.neuropharm.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 53.Hoffman A.N., Parga A., Paode P.R., Watterson L.R., Nikulina E.M., Hammer R.P., Jr., Conrad C.D. Chronic stress enhanced fear memories are associated with increased amygdala zif268 mRNA expression and are resistant to reconsolidation. Neurobiol Learn Mem. 2015;120:61–68. doi: 10.1016/j.nlm.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tronson N.C., Taylor J.R. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- 55.Rescorla R.A. Effect of US habituation following conditioning. J Comp Physiol Psychol. 1973;82:137–143. doi: 10.1037/h0033815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.