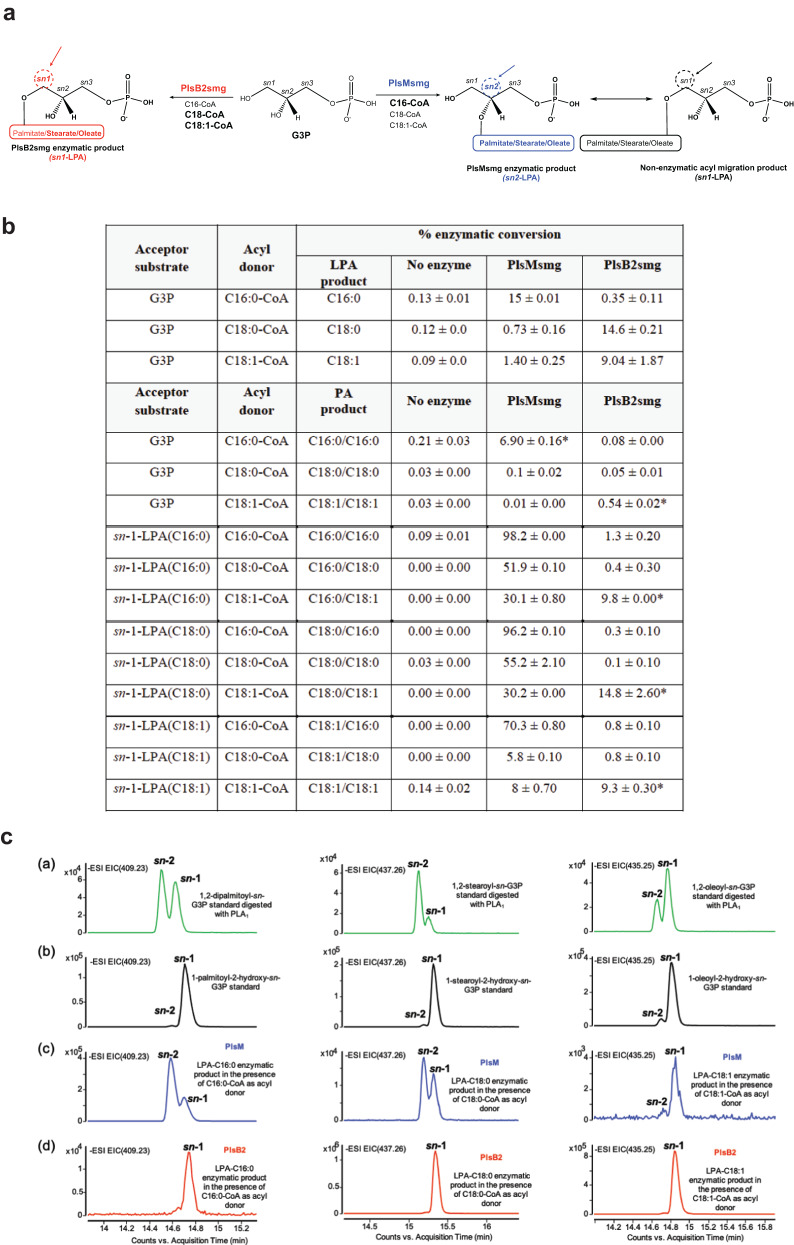
Fig. 3. G3P and LPA acyltransferase activity of PlsMsmg and PlsB2smg.
a Schematic representation of PlsMsmg and PlsB2smg acyltransferase activities in the presence of G3P and various acyl-CoA donors. The arrows point to the positions at which acyl groups are transferred by the two enzymes. Preferred acyl donors in each reaction are in bold letters. b Percentage enzymatic conversion of G3P and various 1-acyl-2-hydroxy-sn-G3P (sn-1-LPAs) to their corresponding LPA and PA products by PlsMsmg and PlsB2smg. Peak areas for substrates and enzymatic products were obtained from the integration of extracted ion chromatograms (EICs) and used to calculate percentage substrate conversion. The LPA and PA products reported for the assays that used G3P as the acceptor substrate are from the same reactions. Values for LPA products include both sn-1 and sn-2-LPAs to take into consideration the spontaneous transmigration of acyl chains. Assays were run as described under Methods for 1 h at 37 °C. The results shown are the means ± standard deviations of duplicate assays and are representative of at least two independent experiments using different enzyme preparations. Asterisks denote products resulting from the spontaneous transmigration of acyl chains between positions sn-1 and sn-2 as detailed in the text and Fig. S7. c G3P acyltransferase activity of PlsMsmg and PlsB2smg. (a) EICs showing LPAs generated from the enzymatic digestion of authentic phosphatidic acid (PA) standards (1,2-dipalmitoyl-sn-G3P, 1,2-stearoyl-sn-G3P and 1-palmitoyl-2-oleoyl-sn-G3P) with phospholipase A1 and analyzed by LC/MS. (b) EICs showing authentic standards of sn-1-palmitoyl-sn-2-hydroxy-G3P, sn-1-stearoyl-sn-2-hydroxy-G3P, and sn-1-oleoyl-sn-2-hydroxy-G3P analyzed by LC/MS. (c) EICs of PlsMsmg enzymatic products generated in the presence of G3P as acceptor substrate and C16:0-CoA, C18:0-CoA and C18:1-CoA as acyl donors. (d) EICs of PlsB2smg enzymatic products generated in the presence of G3P as acceptor substrate and C16:0-CoA, C18:0-CoA and C18:1-CoA as acyl donors. Non-radiolabeled PlsMsmg and PlsB2smg enzyme assays were run as described under Methods. The results shown for both enzymes are representative of at least two independent experiments using different enzyme preparations.