This study attempts to determine if maternal mRNA COVID-19 vaccination during pregnancy is associated with adverse newborn and early infant outcomes.
Key Points
Question
Is maternal messenger RNA (mRNA) COVID-19 vaccination during pregnancy associated with adverse newborn and early infant outcomes?
Findings
In this population-based cohort study of 142 006 live births in Ontario, Canada, maternal mRNA COVID-19 vaccination during pregnancy was associated with lower risks of severe neonatal morbidity, neonatal death, and neonatal intensive care unit admission and no increase in neonatal readmission or hospital admission up to age 6 months, compared with no maternal COVID-19 vaccination before delivery.
Meaning
Maternal mRNA COVID-19 vaccination during pregnancy was not associated with increased adverse newborn and early infant outcomes and may be protective against adverse newborn outcomes.
Abstract
Importance
The study team previously showed that maternal mRNA COVID-19 vaccination during pregnancy confers protection against SARS-CoV-2 infection and COVID-19–related hospital admission in newborns and young infants. In this study, the study team evaluated newborn and early infant safety outcomes following maternal messenger RNA (mRNA) COVID-19 vaccination during pregnancy, for which there is limited comparative epidemiological evidence.
Objective
To determine if maternal mRNA COVID-19 vaccination during pregnancy is associated with adverse newborn and early infant outcomes.
Design, Setting, and Participants
This population-based retrospective cohort study took place in Ontario, Canada, using multiple linked health administrative databases. Singleton live births with an expected delivery date between May 1, 2021, and September 2, 2022, were included. Data were analyzed from January 2023 through March 2023.
Exposure
Maternal mRNA COVID-19 vaccination (1 or more doses) during pregnancy
Main Outcomes and Measures
Severe neonatal morbidity (SNM), neonatal death, neonatal intensive care unit (NICU) admission, neonatal readmission, and hospital admission up to 6 months of age. The study team calculated inverse probability of treatment weighted risk ratios (RRs) and fit weighted Cox proportional hazards regression models comparing outcomes in infants of mothers who received COVID-19 vaccination during pregnancy with those who received no COVID-19 vaccine doses before delivery.
Results
In total, 142 006 infants (72 595 male [51%]; mean [SD] gestational age at birth, 38.7 [1.7] weeks) were included; 85 670 were exposed to 1 or more COVID-19 vaccine doses in utero (60%). Infants of vaccinated mothers had lower risks of SNM (vaccine exposed 7.3% vs vaccine unexposed 8.3%; adjusted RR [aRR], 0.86; 95% CI, 0.83-0.90), neonatal death (0.09% vs 0.16%; aRR, 0.47; 95% CI, 0.33-0.65), and NICU admission (11.4% vs 13.1%; aRR, 0.86; 95% CI, 0.83-0.89). There was no association between maternal vaccination during pregnancy and neonatal readmission (5.5% vs 5.1%; adjusted hazard ratio, 1.03; 95% CI, 0.98-1.09) or 6-month hospital admission (8.4% vs 8.1%; adjusted hazard ratio, 1.01; 95% CI, 0.96-1.05).
Conclusions and Relevance
In this population-based cohort study in Ontario, Canada, maternal mRNA COVID-19 vaccination during pregnancy was associated with lower risks of SNM, neonatal death, and NICU admission. In addition, neonatal and 6-month readmissions were not increased in infants of mothers vaccinated during pregnancy.
Introduction
Pregnant women have an increased risk of severe COVID-19 compared with their nonpregnant counterparts and COVID-19 during pregnancy has been associated with fetal and neonatal morbidity and mortality.1,2 Vaccination is routinely recommended to protect pregnant women and their newborns from acute respiratory tract infections, such as influenza and pertussis, and pregnant women are designated a priority group for COVID-19 vaccination in multiple countries.3 Observational studies have demonstrated that the immunogenicity and effectiveness of COVID-19 vaccines are similar in pregnant and nonpregnant populations.4,5 Moreover, COVID-19 vaccination during pregnancy confers protection to infants during the first months of life.6,7 Increased rates of adverse pregnancy or perinatal outcomes, including spontaneous abortion, stillbirth, and preterm birth, following mRNA COVID-19 vaccination during pregnancy have not been observed in epidemiological studies.8,9,10
Despite this evidence, vaccine coverage of pregnant women has been lower than in nonpregnant women of reproductive age in many regions, especially among younger women and women living in areas with higher socioeconomic deprivation.11,12 Uncertainty about vaccine safety for the infant is one of the most frequently reported reasons for lack of intent to get vaccinated during pregnancy.13,14 Data from large populations on infant outcomes following exposure to COVID-19 vaccines in utero are of high importance and could bolster vaccine confidence in pregnant women but are limited at this stage. Several studies have examined the association between COVID-19 vaccination during pregnancy and admission to the neonatal intensive care unit (NICU) and neonatal respiratory complications,15,16,17,18,19,20,21 but few have assessed a broader range of important neonatal outcomes21,22 and only 1, to our knowledge, has reported safety outcomes for infants beyond the neonatal period.22 To address this knowledge gap, we sought to determine if maternal mRNA COVID-19 vaccination during pregnancy was associated with an increased risk of adverse newborn and early infant outcomes.
Methods
Study Design, Setting, and Participants
We performed a population-based retrospective cohort study of singleton live births with an expected delivery date between May 1, 2021, and September 2, 2022, in Ontario, Canada’s most populous province, with approximately 14.7 million residents and 140 000 births each year.23,24 We excluded infants with a gestational age less than 20 weeks or birth weight less than 500 g, infants of mothers who were not continuously eligible for Ontario health insurance during the year preceding pregnancy, infants with incomplete birth records or records that could not be linked to databases, and infants of mothers younger than 12 years or older than 50 years. We also excluded infants of mothers who received a viral vector-based or non–Health Canada–approved COVID-19 vaccine before delivery. We report the study according to the RECORD (Reporting of Studies Conducted Using Observational Routinely-Collected Health Data) guidelines for observational studies.25
Data Sources
We used data from multiple health administrative databases, which were linked with unique coded identifiers and analyzed at ICES (formerly the Institute for Clinical Evaluative Sciences), an independent, nonprofit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement. For details, see eTable 1 in Supplement 1.
We identified maternal-newborn pairs using the MOMBABY database, which deterministically links hospital delivery records of mothers with the corresponding newborn records from the Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD).26 Hospital births represent more than 98% of births in Ontario27 and more than 99% of live hospital birth records are successfully linked in MOMBABY.26
Exposure
We defined maternal COVID-19 vaccination as receipt of 1 or more messenger RNA (mRNA) COVID-19 vaccine dose(s) between the estimated date of conception and 1 day before birth. We estimated the date of conception from gestational age at birth, which is derived from first or second trimester ultrasound for more than 95% of births in Ontario.28 Mothers were considered unvaccinated if they received no COVID-19 vaccine doses preconception or at any point during pregnancy. Mothers who received their first vaccine dose postpartum were included in the unvaccinated group. Mothers who received 1 or more COVID-19 vaccine dose(s) preconception but none during pregnancy were excluded.
We obtained information on maternal COVID-19 vaccination from a centralized vaccine registry that contains complete information on all COVID-19 vaccinations in Ontario. Pregnant women were designated a priority group for the primary vaccine series in late April 2021, although vaccines were available to some pregnant women (eg, health care or other frontline workers) in December 2020. Due to vaccine supply constraints, the recommended interval between the first and second dose of the primary vaccine series varied from 3 to 16 weeks and some people received a heterologous mRNA vaccine series. In August 2021, people with immunosuppression were eligible for a third COVID-19 vaccine dose and eligibility expanded to all adults, including pregnant women, in December 2021.
Outcomes
Severe Neonatal Morbidity
We defined severe neonatal morbidity (SNM) using an adaptation of the validated composite Neonatal Adverse Outcome Indicator, which includes 15 diagnoses and 7 procedures during the birth admission or within the first 28 days after birth (eTable 2 in Supplement 1).29,30 Previous population-based studies using linked health administrative databases have demonstrated newborns with SNM have an increased risk of poor neurodevelopment in childhood and decreased survival to age 6 years.31,32 We ascertained diagnostic (International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Canadian version) and procedural (Canadian Classifications of Health Interventions) codes using MOMBABY and CIHI-DAD.
Neonatal Mortality and NICU Admission
We defined neonatal mortality as an infant death during the first 28 days of life.33 We obtained data on mortality from CIHI-DAD and the Registered Persons Database. We identified NICU admissions during the first 28 days of life using special care unit admission and discharge dates in CIHI-DAD.
Neonatal and 6-Month Hospital Readmissions
We defined neonatal and 6-month readmission as a hospital admission in the first 28 days or 6 months of life, respectively. We considered transfers between hospitals to be a single admission. We excluded infants who died during the birth admission and those whose birth admission was longer than 28 days (for neonatal readmissions) or 180 days (for 6-month readmissions), since they were not at risk for readmission during those time periods.
Covariates
We adjusted for multiple covariates potentially associated with maternal vaccination and outcomes using propensity score methods. Covariates included infant sex, calendar month and year of conception, maternal age at infant birth, parity, pre-existing maternal medical conditions (hypertension, heart disease, diabetes, asthma, autoimmune disease, immunosuppression), maternal influenza vaccination during either the 2019 to 2020 or 2020 to 2021 influenza seasons (as a proxy for health behavior), adequacy of prenatal care using the Revised Graduated Prenatal Care Index,34 maternal positive SARS-CoV-2 polymerase chain reaction (PCR) test result during pregnancy, neighborhood-level income quintile, neighborhood-level proportion of the population who self-identify as a visible minority quintile, rural residence, and Public Health Unit region.
Statistical Analysis
We estimated unadjusted and inverse probability of treatment weighted risk ratios (RRs) and 95% CIs for neonatal outcomes using modified Poisson regression models with robust variance estimation to account for uncertainty of the weights and for misspecification of the variance structure of the Poisson models. We fit weighted Cox proportional hazards regression models to estimate hazard ratios (HRs) and 95% CIs for neonatal and 6-month hospital readmission with follow-up time beginning on the day of discharge from the birth admission and continuing until readmission, 28 days of age (for neonatal readmission), 6 months of age (for 6-month readmission), or death, whichever occurred first. We used a robust variance estimator to account for within-participant homogeneity in outcomes induced by weighting.
We derived weights from a propensity score representing the predicted probability of receiving 1 or more COVID-19 vaccine dose(s) during pregnancy.35 To reduce variability induced by extreme weights, we used stabilized weights and trimmed values to the 99th percentile.35 We assessed balance of covariates between the weighted exposure groups using standardized differences with values more than 0.1 indicating potentially clinically important imbalance.35
We carried out several subgroup and sensitivity analyses: (1) we conducted a dose-response analyses for 1, 2, and 3 vaccine doses during pregnancy; (2) we restricted the exposed group to infants of mothers who received 1 or more dose(s) in the first, second, or third trimesters; (3) we assessed outcomes in infants of mothers who received BNT162b2 only, mRNA-1273 only, or heterologous mRNA vaccine products during pregnancy; (4) we limited the unvaccinated group to mothers who received their first COVID-19 vaccine dose postpartum; a previous study in Ontario found this group had baseline characteristics more similar to women vaccinated during pregnancy than those never vaccinated18 and there is no evidence suggesting COVID-19 vaccination postpartum is harmful to breastfed infants36; (5) we excluded infants of mothers with documented SARS-CoV-2 infection during pregnancy; (6) because there are sex differences in the prevalence of congenital anomalies, which are major risk factors for neonatal morbidity and death,37 we tested for effect modification by infant sex; (7) we excluded infants who were born at less than 37 weeks’ gestation; (8) we excluded birth trauma from the Neonatal Adverse Outcome Indicator, since this is unlikely to be impacted by maternal COVID-19 vaccination; (9) we calculated weighted RRs and HRs without trimming extreme weights; and (10) for the neonatal and 6-month readmission outcomes, we excluded infants with a birth admission longer than 7 days.
To assess the sensitivity of results to the effects of residual confounding from unmeasured confounders, we performed a quantitative bias analysis using the array approach described by Schneeweiss.38 Briefly, we modeled 2 potential scenarios for each outcome: (1) a higher prevalence of a variable in vaccinated mothers that decreases the risk of an adverse outcome (ie, a protective confounder; RR, 0.2-0.9) and (2) a lower prevalence of a variable in vaccinated mothers that increases the risk of an adverse outcomes (ie, a harmful confounder; RR, 1.5-5). We assumed a confounder prevalence of 20% in unvaccinated mothers and considered a prevalence range of 20% to 35% and 5% to 20% for a protective and harmful confounder in vaccinated mothers, respectively.30 We expressed the degree of bias as a proportion (ie, percent bias = [(observed RR – bias corrected RR) / (bias corrected RR – 1)] × 100).38 We performed analyses using SAS, version 9.4 (SAS Institute).
Results
Study Population
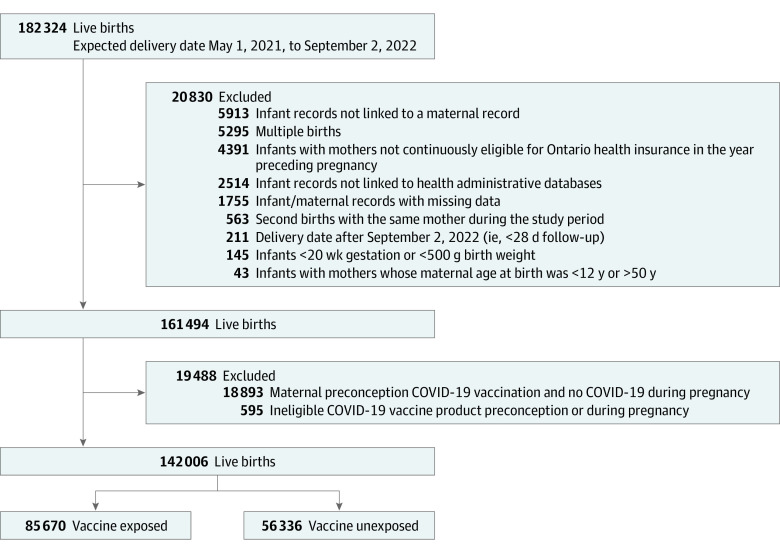
In total, 142 006 infants met eligibility criteria, including 85 670 born to mothers who received 1 or more mRNA COVID-19 vaccine dose(s) during pregnancy (60%) (Figure). Six-month follow-up was available for 112 623 infants (79%). Compared with mothers in the unvaccinated group, those who were vaccinated during pregnancy were more likely to be older than 30 years, nulliparous, influenza vaccine recipients during either of the 2 previous influenza seasons, and residents of urban areas and areas with higher incomes (Table 1). After propensity score weighting, measured baseline characteristics were well balanced across exposure groups with all standardized differences less than 0.10 and with adequate overlap in weighted propensity score distributions (eFigures 1 and 2 in Supplement 1).
Figure. Summary of Cohort Selection.
Table 1. Unweighted Distribution of Baseline Characteristics of Study Population Overall and by Maternal COVID-19 Vaccination Status During Pregnancy.
| Variable | Overall cohort (n = 142 006) | Received ≥1 COVID-19 vaccine dose during pregnancy (n = 85 670) | No COVID-19 vaccine doses before infant birth (n = 56 336) | SDa |
|---|---|---|---|---|
| Duration of follow-up, median (IQR), d | 324 (204-426) | 282 (178-386) | 386 (270-469) | 0.62 |
| ≥6 mo Follow-up after birth (%) | 112 623 (79.3) | 63 892 (74.6) | 48 731 (86.5) | 0.30 |
| Infant sex, No. (%) | ||||
| Female | 69 411 (48.9) | 41 931 (48.9) | 27 480 (48.8) | 0 |
| Male | 72 595 (51.1) | 43 739 (51.1) | 28 856 (51.2) | |
| Gestational age at birth, mean (SD), wk | 38.7 (1.7) | 38.7 (1.6) | 38.6 (1.8) | 0.06 |
| Preterm birth, <37 wk gestation, No. (%) | 8858 (6.2) | 4826 (5.6) | 4032 (7.2) | 0.06 |
| Birth weight, mean (SD), g | 3340 (540) | 3346 (526) | 3330 (561) | 0.03 |
| Low birth weight, <2500 g, No. (%) | 7569 (5.3) | 4139 (4.8) | 3430 (6.1) | 0.06 |
| Birth admission length of stay, median (IQR), db | 1 (1-2) | 1 (1-2) | 1 (1-2) | 0.01 |
| Birth admission length of stay >3 d,b No./total No. (%) | 16 710/141 861 (11.8) | 9440/85 615 (11.0) | 7270/56 246 (12.9) | 0.06 |
| Mother’s age, y, No. (%) | ||||
| <25 | 10 964 (7.7) | 4084 (4.8) | 6880 (12.2) | 0.27 |
| 25-29 | 33 027 (23.3) | 17 457 (20.4) | 15 570 (27.6) | 0.17 |
| 30-34 | 57 764 (40.7) | 37 574 (43.9) | 20 190 (35.8) | 0.16 |
| 35-39 | 33 335 (23.5) | 22 288 (26.0) | 11 047 (19.6) | 0.15 |
| ≥40 | 6916 (4.9) | 4267 (5.0) | 2649 (4.7) | 0.01 |
| Mean (SD) | 31.7 (4.8) | 32.3 (4.5) | 30.8 (5.3) | 0.31 |
| Estimated month-year of conception, No. (%) | ||||
| Aug-20 | 8168 (5.8) | 1090 (1.3) | 7078 (12.6) | 0.46 |
| Sep-20 | 10 462 (7.4) | 4168 (4.9) | 6294 (11.2) | 0.23 |
| Oct-20 | 11 225 (7.9) | 5585 (6.5) | 5640 (10.0) | 0.13 |
| Nov-20 | 10 848 (7.6) | 5665 (6.6) | 5183 (9.2) | 0.10 |
| Dec-20 | 11 111 (7.8) | 6138 (7.2) | 4973 (8.8) | 0.06 |
| Jan-21 | 10 837 (7.6) | 6300 (7.4) | 4537 (8.1) | 0.03 |
| Feb-21 | 9386 (6.6) | 5836 (6.8) | 3550 (6.3) | 0.02 |
| Mar-21 | 9487 (6.7) | 6207 (7.2) | 3280 (5.8) | 0.06 |
| Apr-21 | 8959 (6.3) | 6314 (7.4) | 2645 (4.7) | 0.11 |
| May-21 | 8884 (6.3) | 6505 (7.6) | 2379 (4.2) | 0.14 |
| Jun-21 | 8314 (5.9) | 6345 (7.4) | 1969 (3.5) | 0.17 |
| Jul-21 | 7788 (5.5) | 5831 (6.8) | 1957 (3.5) | 0.15 |
| Aug-21 | 7084 (5.0) | 5251 (6.1) | 1833 (3.3) | 0.14 |
| Sep-21 | 6571 (4.6) | 4992 (5.8) | 1579 (2.8) | 0.15 |
| Oct-21 | 6153 (4.3) | 4620 (5.4) | 1533 (2.7) | 0.14 |
| Nov-21 | 5249 (3.7) | 3789 (4.4) | 1460 (2.6) | 0.10 |
| Dec-21 | 1480 (1.0) | 1034 (1.2) | 446 (0.8) | 0.04 |
| Cesarean delivery, No. (%) | 45 346 (31.9) | 27 884 (32.5) | 17 462 (31.0) | 0.03 |
| Nulliparous, No. (%) | 72 599 (51.1) | 45 778 (53.4) | 26 821 (47.6) | 0.12 |
| Prepregnancy maternal comorbidities, No. (%) | ||||
| Diabetes | 6508 (4.6) | 4478 (5.2) | 2030 (3.6) | 0.08 |
| Hypertension | 2951 (2.1) | 1874 (2.2) | 1077 (1.9) | 0.02 |
| Heart disease | 835 (0.6) | 541 (0.6) | 294 (0.5) | 0.01 |
| Asthma | 24 008 (16.9) | 14 514 (16.9) | 9494 (16.9) | 0 |
| Autoimmune disease | 3263 (2.3) | 2124 (2.5) | 1139 (2.0) | 0.03 |
| Maternal immunosuppression,c No. (%) | 1817 (1.3) | 1030 (1.2) | 787 (1.4) | 0.02 |
| Prenatal care index,d No. (%) | ||||
| No care | 12 896 (9.1) | 7161 (8.4) | 5735 (10.2) | 0.06 |
| Intensive | 1140 (0.8) | 726 (0.8) | 414 (0.7) | 0.01 |
| Adequate | 20 783 (14.6) | 13 117 (15.3) | 7666 (13.6) | 0.05 |
| Intermediate | 75 778 (53.4) | 47 298 (55.2) | 28 480 (50.6) | 0.09 |
| Inadequate | 31 409 (22.1) | 17 368 (20.3) | 14 041 (24.9) | 0.11 |
| Maternal influenza vaccination,e, fNo. (%) | 38 402 (27.0) | 29 774 (34.8) | 8628 (15.3) | 0.46 |
| Maternal positive SARS-CoV-2 PCR test, No. (%) | ||||
| Prepregnancy | 3851 (2.7) | 2457 (2.9) | 1394 (2.5) | 0.02 |
| During pregnancy | 10 472 (7.4) | 6249 (7.3) | 4223 (7.5) | 0.01 |
| Postpartum | 5371 (3.8) | 2721 (3.2) | 2650 (4.7) | 0.08 |
| Neighborhood income quintile,g,h No. (%) | ||||
| 1 (Lowest) | 27 618 (19.4) | 14 105 (16.5) | 13 513 (24.0) | 0.19 |
| 2 | 27 890 (19.6) | 16 047 (18.7) | 11 843 (21.0) | 0.06 |
| 3 | 31 099 (21.9) | 18 893 (22.1) | 12 206 (21.7) | 0.01 |
| 4 | 30 419 (21.4) | 19 497 (22.8) | 10 922 (19.4) | 0.08 |
| 5 (Highest) | 24 980 (17.6) | 17 128 (20.0) | 7852 (13.9) | 0.16 |
| Visible minority quintile,g,i No. (%) | ||||
| 1 (Lowest) | 21 437 (15.1) | 11 556 (13.5) | 9881 (17.5) | 0.11 |
| 2 | 22 945 (16.2) | 13 442 (15.7) | 9503 (16.9) | 0.03 |
| 3 | 25 111 (17.7) | 15 947 (18.6) | 9164 (16.3) | 0.06 |
| 4 | 32 532 (22.9) | 20 974 (24.5) | 11 558 (20.5) | 0.10 |
| 5 (Highest) | 39 981 (28.2) | 23 751 (27.7) | 16 230 (28.8) | 0.02 |
| Public Health Unit region, No. (%) | ||||
| Central East | 8991 (6.3) | 5025 (5.9) | 3966 (7.0) | 0.05 |
| Central West | 29 479 (20.8) | 17 376 (20.3) | 12 103 (21.5) | 0.03 |
| Durham | 8342 (5.9) | 5141 (6.0) | 3201 (5.7) | 0.01 |
| Eastern | 8381 (5.9) | 5013 (5.9) | 3368 (6.0) | 0.01 |
| Northern | 7029 (4.9) | 3857 (4.5) | 3172 (5.6) | 0.05 |
| Ottawa | 10 016 (7.1) | 7158 (8.4) | 2858 (5.1) | 0.13 |
| Peel | 15 490 (10.9) | 8827 (10.3) | 6663 (11.8) | 0.05 |
| South West | 17 541 (12.4) | 9268 (10.8) | 8273 (14.7) | 0.12 |
| Toronto | 26 434 (18.6) | 17 361 (20.3) | 9073 (16.1) | 0.11 |
| York | 10 303 (7.3) | 6644 (7.8) | 3659 (6.5) | 0.05 |
| Rural residence,j No. (%) | 14 581 (10.3) | 7464 (8.7) | 7117 (12.6) | 0.13 |
Abbreviation: PCR, polymerase chain reaction.
Absolute standardized difference. Values greater than 0.10 indicate a potentially clinically important difference in the distribution between groups.
Evaluated in infants discharged alive only (n = 141 861).
Immunosuppression defined as solid organ or stem cell transplant, active cancer, sickle cell anemia, HIV infection, immunosuppressing therapies, and other immune system disorders, derived using the Johns Hopkins ACG System Version 10.0.1 2nd Quarter Release Expanded Diagnostic Cluster for disorders of the immune system.
Adequacy of prenatal care characterized with the Revised-Graduated Prenatal Care Utilization Index.
Maternal influenza vaccination during the 2019 to 2020 and/or 2020 to 2021 influenza seasons.
Dissemination area (400 to 700 residents) level variable.
Household income quintile has variable cutoff values in different cities/census areas to account for cost of living. A dissemination area being in quintile 1 means it is among the lowest 20% of dissemination areas in its city by income.
Percentage of people in the area who self-identified as a visible minority. Census counts for people are randomly rounded up or down to the nearest number divisible by 5, which causes some minor imprecision.
Less than 10 000 residents.
Vaccination Characteristics
Among 85 670 mothers who were vaccinated during pregnancy, 41 621 received 1 dose (48.6%), 42 523 received 2 doses (49.6%), and 1526 received 3 doses during pregnancy (1.8%) (Table 2). Overall, 27 960 received 1 or more dose(s) in the first trimester, 45 901 (54%) received 1 or more dose(s) in the second trimester (33%), and 38 410 received 1 or more dose(s) in the third trimester (45%). A total of 58 533 women received BNT162b2 for all doses during pregnancy (68%).
Table 2. Vaccination Characteristics Among Mothers Who Received at Least 1 COVID-19 Vaccine Dose During Pregnancy.
| Characteristic | ≥1 COVID-19 vaccine doses during pregnancy (n = 85 670), No. (%) |
|---|---|
| No. of COVID-19 vaccines doses during pregnancy | |
| 1 | 41 621 (48.6) |
| 2 | 42 523 (49.6) |
| 3 | 1526 (1.8) |
| At least 1 COVID-19 vaccine dose during trimester 1 | 27 960 (32.6) |
| At least 1 COVID-19 vaccine dose during trimester 2 | 45 901 (53.6) |
| At least 1 COVID-19 vaccine dose during trimester 3 | 38 410 (44.8) |
| Timing of COVID-19 vaccine dose 1 in relation to pregnancy | |
| Preconception | 32 177 (37.6) |
| First trimester | 13 217 (15.4) |
| Second trimester | 23 269 (27.2) |
| Third trimester | 17 007 (19.9) |
| Timing of COVID-19 vaccine dose 2 in relation to pregnancy | |
| Preconception | 20 477 (23.9) |
| First trimester | 13 177 (15.4) |
| Second trimester | 20 653 (24.1) |
| Third trimester | 17 230 (20.1) |
| Postpartum | 10 893 (12.7) |
| Timing of COVID-19 vaccine dose 3 in relation to pregnancy | |
| First trimester | 5454 (6.4) |
| Second trimester | 11 958 (14.0) |
| Third trimester | 9280 (10.8) |
| Postpartum | 30 667 (35.8) |
| BNT162b2 for all COVID-19 vaccine doses during pregnancy | 58 533 (68.3) |
| mRNA-1273 for all COVID-19 vaccine doses during pregnancy | 20 215 (23.6) |
| Heterologous mRNA COVID-19 vaccine doses during pregnancy | 6922 (8.1) |
| Interval between COVID-19 vaccine doses 1 and 2 during pregnancy | |
| <35 d | 10 188 (11.9) |
| 35-55 d | 14 673 (17.1) |
| ≥56 d | 14 499 (16.9) |
| Vaccine dose 1 preconception and/or single dose during pregnancy | 46 310 (54.1) |
| Interval between COVID-19 vaccine doses 2 and 3 during pregnancy | |
| ≤120 d | 567 (0.7) |
| 121-180 d | 3228 (3.8) |
| >180 d | 2305 (2.7) |
| Dose 2 and/or 3 not received during pregnancy | 79 455 (92.9) |
Outcomes
The risks of SNM, neonatal death, and NICU admission were lower in infants of mothers vaccinated during pregnancy compared with unvaccinated mothers (Table 3); after inverse probability of treatment weighting, significantly lower risks persisted (SNM, 7.3% vs 8.3%; adjusted RR [aRR], 0.86; 95% CI, 0.83-0.90; neonatal death, 0.09% vs 0.16%; aRR, 0.47; 95% CI, 0.33-0.65; and NICU admission, 11.4% vs 13.1%; aRR, 0.86; 95% CI, 0.83-0.89). The risk of neonatal readmission among infants of vaccinated and unvaccinated mothers was 5.5% and 5.1%, respectively, which was not statistically significant after adjusting for confounding (adjusted HR, 1.03; 95% CI, 0.98-1.09). Likewise, there was no significant association between maternal vaccination during pregnancy and 6-month readmission (8.2% vs 7.9%; adjusted HR, 1.01; 95% CI, 0.96-1.05). Estimates were similar when the analyses were limited to infants discharged 7 days or less after birth (Table 3). Relative to infants of unvaccinated mothers, a significantly lower proportion of infants of vaccinated mothers were excluded from the analyses of readmission because they died during their birth admission (0.06% vs 0.16%; aRR, 0.36; 95% CI, 0.25-0.52) or because their birth admission was longer than 28 days (0.95% vs 1.5%; aRR, 0.59; 95% CI, 0.53-0.65) or 180 days (0.01% vs 0.04%; aRR, 0.37; 95% CI, 0.17-0.82).
Table 3. Association Between COVID-19 Vaccination During Pregnancy and Neonatal and Infant Outcomes.
| No./total No. (%) | Risk ratio or hazard ratio (95% CI) | |||
|---|---|---|---|---|
| Outcome | Vaccine exposed | Unexposed | Crude | Adjusteda |
| Severe neonatal morbidity | 6229/85 670 (7.3) | 4697/56 336 (8.3) | 0.87 (0.84-0.90) | 0.86 (0.83-0.90) |
| Neonatal death | 74/85 670 (0.09) | 91/56 336 (0.16) | 0.53 (0.39-0.73) | 0.47 (0.33-0.65) |
| Neonatal intensive care unit admission | 9721/85 670 (11.4) | 7391/56 336 (13.1) | 0.86 (0.84-0.89) | 0.86 (0.83-0.89) |
| Neonatal readmission | 4664/84 798 (5.5) | 2820/55 417 (5.1) | 1.08 (1.03-1.13) | 1.03 (0.98-1.09) |
| Discharged within 7 d after birth | 4588/82 581 (5.6) | 2769/53 330 (5.2) | 1.07 (1.02-1.12) | 1.03 (0.97-1.08) |
| Hospital admission up to 6 mo of age | 5361/63 834 (8.4) | 3941/48 625 (8.1) | 1.04 (1.00-1.08) | 1.01 (0.96-1.05) |
| Discharged within 7 d after birth | 5020/61 426 (8.2) | 3611/46 007 (7.9) | 1.04 (1.00-1.09) | 1.01 (0.97-1.06) |
Risk ratios and hazard ratios were adjusted using stabilized inverse probability of treatment weighting, trimmed at the 99th percentile.
In additional analyses, results were largely unchanged by number of vaccine doses during pregnancy, trimester of vaccination, vaccine product, or infant sex. Likewise, limiting the unvaccinated group to infants of mothers who received their first COVID-19 vaccine dose during the postpartum period or excluding preterm infants or infants of mothers who had a positive SARS-CoV-2 PCR test during pregnancy produced similar results (eTable 3 in Supplement 1).
Sensitivity Analyses
Sensitivity analyses examining the potential impact of unmeasured confounding indicated that both hypothetical scenarios (ie, a higher prevalence of a protective confounder or a lower prevalence of a harmful confounder in the vaccine exposed group) would have biased the original estimates downward for all outcomes (eTable 4 and eFigure 3 in Supplement 1). Despite this, most bias-corrected estimates for SNM, neonatal death, and NICU admission remained less than 1 after accounting for hypothetical unmeasured confounding. The most extreme bias-corrected HRs for neonatal and 6-month readmission (ie, requiring a 15% lower prevalence of a harmful confounder in the vaccinated group with a confounder-outcome HR = 5) were 1.55 and 1.52, respectively.
Discussion
Principal Findings
In this population-based study examining outcomes among more than 140 000 live births in Ontario, Canada, maternal mRNA COVID-19 vaccination during pregnancy was not associated with adverse newborn outcomes and instead was associated with lower risks of SNM, neonatal death, and NICU admission. In addition, neonatal and 6-month readmissions were not increased in infants of mothers vaccinated during pregnancy. Results were mostly unchanged in additional analyses based on the number of vaccine doses during pregnancy, trimester of vaccination, and mRNA vaccine product. Moreover, limiting the unexposed group to infants of mothers who received their first COVID-19 vaccine dose in the postpartum period and excluding infants of mothers with documented SARS-CoV-2 infection during pregnancy produced similar findings.
Comparison With Other Studies
Our results are consistent with previously published studies that reported no increased risk of NICU admission among infants of mothers who received 1 or more mRNA COVID-19 vaccine dose(s) during pregnancy compared with infants of unvaccinated mothers.16,17,18,19,20,21 Most of these studies did not include sufficient numbers of infants to produce precise estimates.16,19,20,21 Moreover, less than 25% of mothers were vaccinated in many studies,16,17,18,19,20 compared with 60% in the present study and first trimester exposures were either absent19,21 or underrepresented.16,17,18,20 Our results are also comparable with a study from 2 university-affiliated hospitals in Israel (n = 1750) that assessed the association between third trimester maternal BNT162b2 vaccination and a composite neonatal adverse outcome indicator, which had overlap with the SNM indicator we used.21 Similar to our finding, neonatal adverse outcomes were lower among infants of vaccinated mothers (8% vs 11%; adjusted odds ratio, 0.50; 95% CI, 0.36-0.74).21 An Israeli population-based cohort study of more than 24 000 live births reported no association between maternal vaccination and neonatal readmission (aRR, 0.99; 95% CI, 0.88-1.12), postneonatal readmission (aRR, 0.95; 95% CI, 0.84-1.07), or death up to 7 months (aRR, 0.84; 95% CI, 0.43-1.73).22
Interpretation
This study contributes to the scientific literature in 2 important ways. First, it provides further reassurance on the safety of maternal mRNA COVID-19 vaccination during all trimesters of pregnancy for newborns and infants. Second, it adds to existing evidence15,16,17,18,19,20,21,22 suggesting that not only are there no apparent increased risks of the adverse neonatal and early infant outcomes evaluated in this study following maternal COVID-19 vaccination during pregnancy, but there may be potential benefits. While it is possible that residual confounding might have biased estimates away from the null, as suggested by our quantitative bias analyses, except for the most extreme scenarios, bias-corrected estimates for SNM, neonatal death, and NICU admission remained less than 1 after accounting for hypothetical unmeasured confounding. Lower risks of adverse neonatal outcomes among infants of vaccinated mothers would be consistent with the well-documented association between severe COVID-19 during pregnancy and increased neonatal morbidity,1,2 together with evidence suggesting that COVID-19 vaccination reduces the risk of severe COVID-19 in pregnant populations.5 Documented SARS-CoV-2 infection during pregnancy was included in propensity scores and estimates were unchanged after excluding infants of mothers with documented infection during pregnancy, suggesting it is unlikely to fully explain the apparent reduced risk of adverse neonatal outcomes in vaccinated mothers. Downstream impacts on newborn outcomes due to a protective effect of vaccination against SARS-CoV-2-associated placental damage or other immunological responses to infection of all severities, including those that were undocumented, is also biologically plausible.
By contrast, we found that maternal COVID-19 vaccination during pregnancy was associated with a slightly increased risk of neonatal readmission in unadjusted analyses. We hypothesize the observed increase was due to depletion of susceptibles39; this is supported by the finding that a small proportion of infants in the unexposed group were more likely to be excluded from analyses of readmission either because they died during the birth admission or because their birth admission was longer than 28 days. In the weighted primary analysis and most subgroup/sensitivity analyses, the association between maternal vaccination and neonatal readmission was no longer significant and there was no increase in hospital readmission up to age 6 months.
Strengths and Limitations
Strengths of this study include the use of deterministically linked, population-based databases within a universal health care system, which allowed us to identify all hospital births in Ontario during the study period, thereby limiting potential selection bias. Detailed information on vaccination status through a centralized COVID-19 vaccine registry minimized the potential for exposure misclassification bias. The use of early ultrasound assessment for most births in Ontario assured accurate gestational timing of maternal vaccination. We assessed readmission up to age 6 months, which is among the longest reported follow-up to date, for infants exposed to COVID-19 vaccines in utero.
Our study also has limitations. First, although we achieved good balance in the distribution of baseline covariates in weighted analyses, propensity scores were limited to variables available in study databases. We did not have information on maternal body mass index, tobacco use, or breastfeeding. However, our results were robust to sensitivity analyses designed to account for residual confounding. Second, our analyses were restricted to live births. Assessment of outcomes in live-born infants only would miss effects of vaccination on spontaneous abortions and stillbirths, if there are any, potentially resulting in live-birth bias.40 Epidemiological studies, including data from Ontario, have not reported an elevated risk of spontaneous abortion or stillbirth in women who received mRNA COVID-19 vaccines during pregnancy, with some studies finding reductions in stillbirth risk.8,9,10 Third, despite the large sample size, our study might have been underpowered to rule out small associations for rare events, such as neonatal mortality. Moreover, we observed a large relative risk reduction in neonatal mortality even though the absolute difference in the number of neonatal deaths between exposure groups was small. As neonatal mortality has declined in recent decades, severe morbidity has been suggested as a more relevant outcome and use of a composite morbidity indicator helps to overcome issues with power, as well as under ascertainment of individual conditions or procedures.29
Conclusions
Our study substantially expands on existing evidence of the safety of maternal mRNA vaccination during pregnancy for newborns and young infants. COVID-19 vaccination during pregnancy was associated with lower risks of SNM, neonatal death, and NICU admission and no increases in neonatal and 6-month readmissions.
eFigure 1. Standardized differences for baseline characteristics before and after weighting using stabilized inverse probability of treatment weights
eFigure 2. Overlap in propensity score distributions by exposure group before and after weighting using stabilized inverse probability of treatment weights
eFigure 3. Bias analyses for severe neonatal morbidity: percent bias as a function of the prevalence of the hypothetical confounder in the vaccine exposed group
eTable 1. Linked health administrative databases
eTable 2. Severe neonatal morbidity indicator
eTable 3. Additional newborn and infant outcome analyses
eTable 4. Array approach sensitivity analyses for unmeasured potential confounders
Data sharing statement
References
- 1.Allotey J, Stallings E, Bonet M, et al. ; for PregCOV-19 Living Systematic Review Consortium . Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. doi: 10.1136/bmj.m3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8):817-826. doi: 10.1001/jamapediatrics.2021.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zavala E, Krubiner CB, Jaffe EF, et al. Global disparities in public health guidance for the use of COVID-19 vaccines in pregnancy. BMJ Glob Health. 2022;7(2):e007730. doi: 10.1136/bmjgh-2021-007730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray KJ, Bordt EA, Atyeo C, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225(3):303.e1-303.e17. doi: 10.1016/j.ajog.2021.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldshtein I, Nevo D, Steinberg DM, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326(8):728-735. doi: 10.1001/jama.2021.11035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jorgensen SCJ, Hernandez A, Fell DB, et al. ; Canadian Immunization Research Network (CIRN) Provincial Collaborative Network (PCN) Investigators . Maternal mRNA covid-19 vaccination during pregnancy and delta or omicron infection or hospital admission in infants: test negative design study. BMJ. 2023;380:e074035. doi: 10.1136/bmj-2022-074035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halasa NB, Olson SM, Staat MA, et al. ; Overcoming Covid-19 Investigators . Maternal vaccination and risk of hospitalization for COVID-19 among infants. N Engl J Med. 2022;387(2):109-119. doi: 10.1056/NEJMoa2204399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zauche LH, Wallace B, Smoots AN, et al. ; CDC v-safe Covid-19 Pregnancy Registry Team . Receipt of mRNA COVID-19 vaccines and risk of spontaneous abortion. N Engl J Med. 2021;385(16):1533-1535. doi: 10.1056/NEJMc2113891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magnus MC, Gjessing HK, Eide HN, Wilcox AJ, Fell DB, Håberg SE. Covid-19 Vaccination during pregnancy and first-trimester miscarriage. N Engl J Med. 2021;385(21):2008-2010. doi: 10.1056/NEJMc2114466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fell DB, Dimanlig-Cruz S, Regan AK, et al. Risk of preterm birth, small for gestational age at birth, and stillbirth after covid-19 vaccination during pregnancy: population based retrospective cohort study. BMJ. 2022;378:e071416. doi: 10.1136/bmj-2022-071416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fell DB, Török E, Sprague AE, et al. Temporal trends and determinants of COVID-19 vaccine coverage and series initiation during pregnancy in Ontario, Canada, December 2020 to December 2021: a population-based retrospective cohort study. Vaccine. 2023;41(10):1716-1725. doi: 10.1016/j.vaccine.2023.01.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Razzaghi H, Meghani M, Pingali C, et al. COVID-19 vaccination coverage among pregnant women during pregnancy—eight integrated health care organizations, United States, December 14, 2020-May 8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(24):895-899. doi: 10.15585/mmwr.mm7024e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramlawi S, Muldoon KA, Dunn SI, et al. Worries, beliefs and factors influencing perinatal COVID-19 vaccination: a cross-sectional survey of preconception, pregnant and lactating individuals. BMC Public Health. 2022;22(1):2418. doi: 10.1186/s12889-022-14617-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skjefte M, Ngirbabul M, Akeju O, et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021;36(2):197-211. doi: 10.1007/s10654-021-00728-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wainstock T, Yoles I, Sergienko R, Sheiner E. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine. 2021;39(41):6037-6040. doi: 10.1016/j.vaccine.2021.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, Swift M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obstet Gynecol MFM. 2021;3(6):100467. doi: 10.1016/j.ajogmf.2021.100467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnus MC, Örtqvist AK, Dahlqwist E, et al. Association of SARS-CoV-2 vaccination during pregnancy with pregnancy outcomes. JAMA. 2022;327(15):1469-1477. doi: 10.1001/jama.2022.3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fell DB, Dhinsa T, Alton GD, et al. Association of COVID-19 vaccination in pregnancy with adverse peripartum outcomes. JAMA. 2022;327(15):1478-1487. doi: 10.1001/jama.2022.4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blakeway H, Prasad S, Kalafat E, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226(2):236 e231-236 e214. doi: 10.1016/j.ajog.2021.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibroci E, Liu X, Lieb W, et al. Impact of prenatal COVID-19 vaccination on delivery and neonatal outcomes: results from a New York City cohort. Vaccine. 2023;41(3):649-656. doi: 10.1016/j.vaccine.2022.09.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rottenstreich M, Sela HY, Rotem R, Kadish E, Wiener-Well Y, Grisaru-Granovsky S. Covid-19 vaccination during the third trimester of pregnancy: rate of vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study. BJOG. 2022;129(2):248-255. doi: 10.1111/1471-0528.16941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldshtein I, Steinberg DM, Kuint J, et al. Association of BNT162b2 COVID-19 vaccination during pregnancy with neonatal and early infant outcomes. JAMA Pediatr. 2022;176(5):470-477. doi: 10.1001/jamapediatrics.2022.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Statistics Canada . Table 13-10-0414-01 Live births, by place of residence of mother. 10.25318/1310041401-eng September 19, 2023. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310041401 [DOI]
- 24.Statistics Canada . Table 17-10-0005-01 Population estimates on July 1st, by age and sex. Accessed September 19, 2023. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501
- 25.Benchimol EI, Smeeth L, Guttmann A, et al. ; RECORD Working Committee . The reporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. doi: 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MOMBABY . ICES data dictionary (ICES, Toronto). Accessed September 12, 2023. https://datadictionary.ices.on.ca/Applications/DataDictionary/Library.aspx?Library=MOMBABY
- 27.Statistics Canada . Table 13-10-0429-01 Live births and fetal deaths (stillbirths), by place of birth (hospital or non-hospital). Accessed September 19, 2023. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310042901
- 28.You JJ, Alter DA, Stukel TA, et al. Proliferation of prenatal ultrasonography. CMAJ. 2010;182(2):143-151. doi: 10.1503/cmaj.090979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lain SJ, Algert CS, Nassar N, Bowen JR, Roberts CL. Incidence of severe adverse neonatal outcomes: use of a composite indicator in a population cohort. Matern Child Health J. 2012;16(3):600-608. doi: 10.1007/s10995-011-0797-6 [DOI] [PubMed] [Google Scholar]
- 30.Fakhraei R, Crowcroft N, Bolotin S, et al. Obstetric and perinatal health outcomes after pertussis vaccination during pregnancy in Ontario, Canada: a retrospective cohort study. CMAJ Open. 2021;9(2):E349. doi: 10.9778/cmajo.20200239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bentley JP, Schneuer FJ, Lain SJ, Martin AJ, Gordon A, Nassar N. Neonatal morbidity at term, early child development, and school performance: a population study. Pediatrics. 2018;141(2):e20171726. doi: 10.1542/peds.2017-1726 [DOI] [PubMed] [Google Scholar]
- 32.Stephens AS, Lain SJ, Roberts CL, Bowen JR, Nassar N. Association of gestational age and severe neonatal morbidity with mortality in early childhood. Paediatr Perinat Epidemiol. 2016;30(6):583-593. doi: 10.1111/ppe.12323 [DOI] [PubMed] [Google Scholar]
- 33.Irvine B, Dzakpasu S, León JA. Perinatal health indicators 2013: a surveillance report by the Public Health Agency of Canada’s Perinatal Surveillance System. Health Promot Chronic Dis Prev Can. 2015;35(1):23-24. doi: 10.24095/hpcdp.35.1.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexander GR, Kotelchuck M. Quantifying the adequacy of prenatal care: a comparison of indices. Public Health Rep. 1996;111(5):408-418. [PMC free article] [PubMed] [Google Scholar]
- 35.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661-3679. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Institutes of Health . Drugs and lactation database. Accessed September 12, 2023. https://www.ncbi.nlm.nih.gov/books/NBK565969/
- 37.Lary JM, Paulozzi LJ. Sex differences in the prevalence of human birth defects: a population-based study. Teratology. 2001;64(5):237-251. doi: 10.1002/tera.1070 [DOI] [PubMed] [Google Scholar]
- 38.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15(5):291-303. doi: 10.1002/pds.1200 [DOI] [PubMed] [Google Scholar]
- 39.Fireman B, Gruber S, Zhang Z, et al. Consequences of depletion of susceptibles for hazard ratio estimators based on propensity scores. Epidemiology. 2020;31(6):806-814. doi: 10.1097/EDE.0000000000001246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raz R, Kioumourtzoglou MA, Weisskopf MG. Live-birth bias and observed associations between air pollution and autism. Am J Epidemiol. 2018;187(11):2292-2296. doi: 10.1093/aje/kwy172 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Standardized differences for baseline characteristics before and after weighting using stabilized inverse probability of treatment weights
eFigure 2. Overlap in propensity score distributions by exposure group before and after weighting using stabilized inverse probability of treatment weights
eFigure 3. Bias analyses for severe neonatal morbidity: percent bias as a function of the prevalence of the hypothetical confounder in the vaccine exposed group
eTable 1. Linked health administrative databases
eTable 2. Severe neonatal morbidity indicator
eTable 3. Additional newborn and infant outcome analyses
eTable 4. Array approach sensitivity analyses for unmeasured potential confounders
Data sharing statement