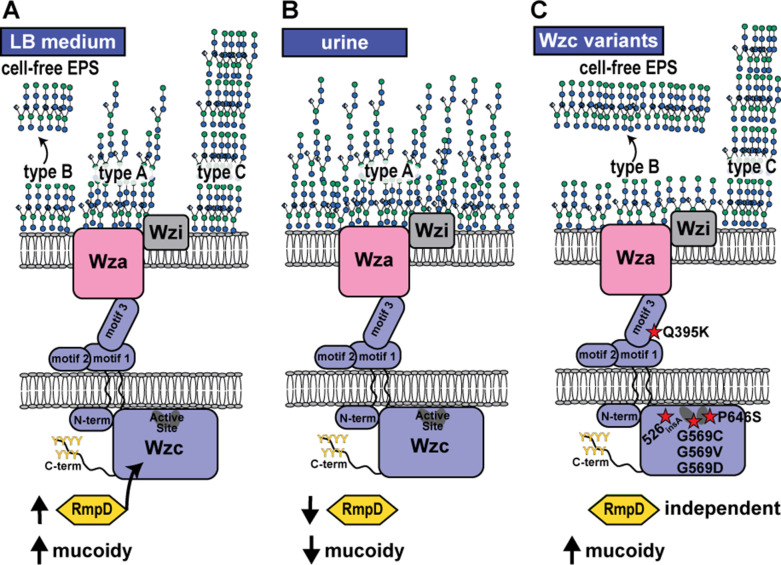
Fig 3.
Model of K. pneumoniae control of capsule biosynthesis and mucoidy. Wzc is an inner membrane tyrosine kinase that regulates capsule (CPS) biosynthesis and extrusion. (A) When cultured in LB medium, K. pneumoniae KPPR1 presents as hypermucoid, which resists sedimentation. In this condition, three distinct cell-associated CPS species with different chain length properties are detected. Type A is a broad band with diverse chain lengths. Type B is a narrow band representing consistent chain lengths; this is the species also found as cell-free EPS. Type C is an ultra-high molecular weight CPS species. (B) Urine down-regulates rmpD transcription and results in lower mucoidy. Culturing K. pneumoniae in urine results in tightly cell-associated type A CPS production. (C) Six Wzc variants were identified here that overcome urine-induced mucoidy suppression independent of RmpD. The Wzc mutations are marked with a red star, where the dark ovals represent Walker A and B motifs. WzcQ395K is localized to the periplasmic motif 3, predicted to interact with the Wza outer membrane protein. WzcG569 is adjacent to the active site tyrosine. WzcP646S is located in the active site Walker B motif. Wzc526insA is located eight residues up-stream of the active site Walker A motif. All mutations result in constitutive production of type B CPS and copious secretion of type B CPS into the supernatant as cell-free EPS. All mutations, except WzcQ395K, also increase production of cell-associate type C CPS, particularly in urine.