Supplemental Digital Content is Available in the Text.
This is a report of the combined human amniotic membrane and macular hole hydrodissection technique for the repair of large macular holes. The shelf created by the macular hole hydrodissection allows for the graft to be secured in place for better adherence to the retinal pigment epithelium.
Key words: macular hole, vitreoretinal surgery, amniotic membrane, graft, retina, surgical technique
Abstract
Purpose:
To describe a combined surgical technique using the macular hole hydrodissection (MHH) with human amniotic membrane for repair of large macular holes.
Methods:
A step-by-step procedure and a surgical video using the combined MHH and human amniotic membrane technique are presented.
Description and technique:
As the first step, the MHH separates the adhesions of the macular hole to the underlying retinal pigment epithelium with a soft-tipped cannula through proportional reflux followed by gentle passive aspiration. The human amniotic membrane graft is marked to identify the nonsticky epithelial side and ensure that the stromal layer (sticky and nonshinny) is facing downward toward the retinal pigment epithelium. The graft is then tucked into the space created with MHH between the macular hole edges and the retinal pigment epithelium with closed forceps to decrease the likelihood of the graft from dislocating postoperatively.
Conclusion:
The MHH in combination with the human amniotic membrane is a practical and effective technique for addressing challenging large macular holes.
We have previously described the macular hole hydrodissection (MHH) technique, which may be beneficial for the repair of large, persistent, and/or chronic macular holes.1 Our findings have suggested that this technique is effective in closing 87% of the most challenging macular holes, with 95% of patients experiencing vision improvement.1 Despite these promising results, for macular holes that are exceedingly large (>800 μm), the use of a graft such as a human amniotic membrane (hAM) is often necessary to ensure complete closure of MHs. One of the earliest subretinal implantations of hAM was performed in rabbits by Rosenfeld et al2 described in 1999. The hAM possess a number of suitable qualities for macular hole repair,3,4 with good anatomical and functional recovery in challenging cases.5,6
The MHH and hAM involve a gentle approach to creation of mobile retinal edges to allow for the hAM to be placed in the correct position. Our group has recently described the good surgical and visual outcomes achieved with MHH and hAM.7 In this study, we provide a detailed step-by-step description of our approach to the use of hAM combined with the MHH for the repair of large macular holes.
Description of Surgical Technique
The combined hAM and MHH technique involves several critical steps to ensure optimal outcomes. As the first step with the MHH, the adhesions of the macular hole to the underlying retinal pigment epithelium (RPE) are separated with a soft-tipped cannula by manipulating the edges of the macular hole (Figure 1). This is achieved through proportional reflux followed by gentle passive aspiration. The space created with MHH between the macular hole edges and the RPE will serve as a shelf for the placement of the hAM. The shelf is essential in decreasing the likelihood of the graft from dislocating postoperatively.
Fig. 1.
Combined hAM and MHH technique for repair of large macular holes. The MHH separates the adhesions of the macular hole to the underlying RPE with a soft-tipped cannula through proportional reflux (A) followed by gentle passive aspiration (B). The hAM graft is prepared using a 1-2 mm punch, and a small dot from a marking pen is used to identify the nonsticky epithelial side to ensure that the stromal layer (sticky and nonshiny) is facing downward toward the RPE (C). The graft is then tucked into the space created with MHH between the macular hole edges and the RPE with closed forceps to decrease the likelihood of the graft from dislocating postoperatively (D). Important consideration when preparing the graft is to trim the graft to the size of the macular hole to avoid grafts that are too small or too large (E).
The hAM graft is then prepared using a 1-mm to 2-mm punch. While preparing the graft, a small dot from a marking pen is used to identify the nonsticky epithelial side of the hAM so it can be identified once placed in the vitreous cavity. This step will ensure that the stromal layer (sticky and nonshiny) is facing downward toward the RPE. When preparing the graft, fine scissors may be used to trim the hAM to a size just larger than the macular hole so the graft will fit in the macular hole although using a 1-mm punch will usually yield the ideal graft size for macular holes under 1,000 µm. Attention should be paid to the diameter of the plug because hAM grafts that are too small or too large will result in grafts that will not attach under the shelf of the macular hole edges or create folds from tissue redundancy, respectively. We use preoperative measurements of the macular hole size on optical coherence tomography to estimate the size of the punch before the surgery date. In our experience, for macular holes larger than 1,000 µm a 2-mm punch can be considered but will often need to be trimmed down.
The hAM is then placed in the vitreous cavity with a fine gripping forceps and tucked into the space created around the macular hole edges. This step can be performed with closed forceps to avoid the hAM from sticking to the teeth of the forceps. A fluid–air exchange is performed, and the eye may be flushed with SF6 gas (20%). A detailed step-by-step summary of our surgical technique is provided in Video 1 (Supplemental Digital Content 1, http://links.lww.com/ICB/A165).
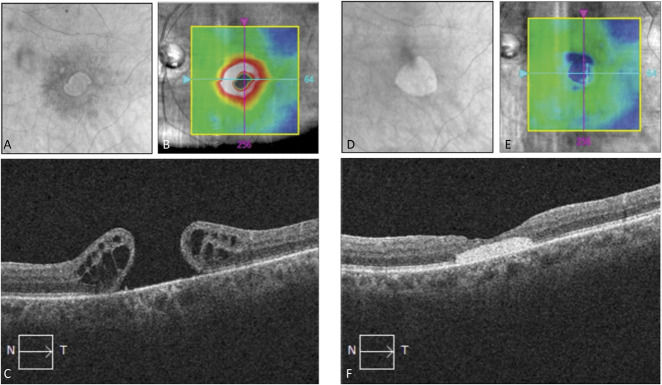
Figure 2 presents a case of a man with a persistent macular hole. His macular hole was measured preoperatively at the aperture at 722 µm and 1,084 µm at the base with a visual acuity of 20/200. He underwent MHH with hAM and achieved complete anatomical closure of the macular hole with a visual acuity of 20/70 at 15 months postoperatively.
Fig. 2.
Optical coherence tomography fundus, ILM-RPE thickness map, and horizontal macular cube scans before (A–C) and after (D–F) macular hole repair with the MHH technique and human amniotic membrane. Postoperative images at 15 months depict a well-positioned human amniotic membrane in the macular area and full macular hole closure.
Discussion
The addition of MHH to transplantation of hAM allows for an easier intraoperative securing of the graft within the macular hole. This additional simple step has been shown to be effective in lysing the adherent edges of chronic macular holes, with good anatomical and vision outcomes,1 and can be easily adapted to the surgeon's routine surgical technique. Another advantage of tucking the hAM plug into the MHH dissected edges is the added security for preventing dislocation of the tissue into the vitreous cavity postoperatively which has been reported in some cases.6 The additional confidence provided by securing the hAM edges in the macular hole also reduces the need for the use of gas tamponade at the conclusion of the surgery. Furthermore, our preoperative labeling of the epithelia layer is a useful step in ensuring the graft adheres in place while helping with the efficiency of finding the correct position intraoperatively.
Similar techniques have been proposed by Huang et al8 who secured the hAM graft by trapping its edge beneath the retina. They also identified the epithelial side of the graft by staining it with Brilliant Blue G preoperatively. In another study, Caporossi et al9 also describe a gentle manipulation and insertion of the hAM into the macular hole in the subretinal space with the sticky layer facing the RPE. Other techniques described involve cutting the hAM graft inside the vitreous cavity with vitreoretinal scissors and then outstretching the graft using two forceps for macular holes measuring at diameters less than 1 mm to avoid folds.6 We believe that the use of the MHH for transplantation of hAM will likely decrease the need for trimming the graft inside the vitreous. The shelf created by MHH also eliminates over manipulation of the hAM to get rid of tissue redundancy because the hAM edges can be neatly tucked under this shelf as much as necessary until it sits flat on the RPE surface.
Rizzo et al5 describe the transplantation of the hAM under fluid or perfluorocarbon into the subretinal space of the macular hole. In our experience, the use of heavy liquid is not needed with the MHH because the edges of hAM graft are securely tucked in the hydrodissected macular hole edges. As such, we only use air exchange and often do not need gas for hAM transplantation with MHH.
It has been shown that RPE cells cultured on hAM maintain retinal homeostasis and improve eye function.10 Previous reports have demonstrated the significance of reattachment of the margins and neuroretinal ingrowth over the hAM plug postoperatively on patient outcomes.5,11 We hypothesize that the stimulation of the macular hole edges and shelf created by overlaying the hydrodissected retina over the hAM using MHH will promote better reattachment of the margins and neuroretinal ingrowth over the hAM.
In this article, we described the combined MHH and hAM transplantation, which takes advantage of the two surgical techniques to allow for an easier securing of the graft for some of the most difficult macular holes encountered. This technique may allow surgeons to create a shelf for more controlled insertion of the hAM graft in place and potentially better adherence by lysing the adherent edges of the recurrent/chronic macular holes.
Footnotes
None of the authors has any financial/conflicting interests to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.retinajournal.com).
Contributor Information
Michael Corrin, Email: m.corrin@utoronto.ca.
John Papanikolaou, Email: johnf.papanikolaou@gmail.com.
Efrem D. Mandelcorn, Email: efrem.mandelcorn@utoronto.ca.
References
- 1.Felfeli T, Mandelcorn ED. Macular hole hydrodissection: surgical technique for the treatment of persistent, chronic, and large macular holes. Retina 2019;39:743–752. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld P, Merritt J, Hernandez E, et al. Subretinal implantation of human amniotic membrane: a rabbit model for the replacement of Bruch's membrane during submacular surgery. Invest Ophthalmol Vis Sci 1999;40:S206. [Google Scholar]
- 3.Ramuta TŽ, Kreft ME. Human amniotic membrane and amniotic membrane–derived cells: how far are we from their use in regenerative and reconstructive urology? Cell Transpl 2018;27:77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdelhakim BAH, Tezel TH. Human amniotic membrane for macular hole surgery. Retin Spec 2021:1–10. [Google Scholar]
- 5.Rizzo S, Caporossi T, Tartaro R, et al. A human amniotic membrane plug to promote retinal breaks repair and recurrent macular hole closure. Retina 2019;39:S95–S103. [DOI] [PubMed] [Google Scholar]
- 6.Caporossi T, Pacini B, Bacherini D, et al. Human amniotic membrane plug to promote failed macular hole closure. Sci Rep 2020;10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bamberger M, Felfeli T, Politis M, et al. Human amniotic membrane plug for chronic or persistent macular holes. Ophthalmol Retina 2022;6(5):431‐433. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y, Tsai D, Wang L, Chen S. Comparison between cryopreserved and dehydrated human amniotic membrane graft in treating challenging cases with macular hole and macular hole retinal detachment. J Ophthalmol 2020:2020:9157518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caporossi T, Pacini B, De Angelis L, et al. Human amniotic membrane to close recurrent, high myopic macular holes it pathologic myopia with axial length of ≥30 mm. Retina 2020;40:1946–1954. [DOI] [PubMed] [Google Scholar]
- 10.Ohno-Matsui K, Ichinose S, Nakahama KI, et al. The effects of amniotic membrane on retinal pigment epithelial cell differentiation. Mol Vis 2005;11:1–10. [PubMed] [Google Scholar]
- 11.Ventre L, Marolo P, Reibaldi M. A human amniotic membrane plug to treat persistent macular hole. Case Rep Ophthalmol 2020;11(2):442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]