Abstract
Objective
This study aims to elucidate the trajectory of quality of life (QoL) over a two-year period after radiotherapy and/or chemotherapy for head and neck cancer (HNC), addressing the gap in long-term QoL information.
Methods
Employing a prospective longitudinal observational design, we tracked 58 HNC patients who underwent radiotherapy and/or chemotherapy, analyzing their QoL using Short-Form 36-Item Health Survey version 2 (SF36v2), the European Organization for Research and Treatment of Cancer quality of life (EORTC-QLQ-C30), and the European Organization for Research and Treatment of Cancer quality of life head and neck-35 (EORTC-QLQ-H&N35) questionnaires for two years post-discharge. The data underwent repeated measures analysis of variance.
Results
Over the two-year follow-up, 10 patients (17.2%) succumbed, and 8 (13.8%) dropped out. SF36v2 physical and role-social component summary scores declined during treatment, requiring 1–2 years for recovery. The mental component summary score remained stable. EORTC-QLQ-30 revealed global health status recovery within one year post-discharge. EORTC-QLQ-H&N35 items like “swallowing,” “senses problems,” “trouble with social eating,” “dry mouth,” “sticky saliva,” “coughing,” and “felt ill” worsened pre-discharge. “Trouble with social contact” improved within a year, while “pain,” “swallowing,” “senses problems,” “trouble with social eating,” and “coughing” improved within two years. “Dry mouth” and “sticky saliva” persisted throughout the two-year follow-up, common symptoms of HNC and treatment side effects.
Conclusions
Recovery of specific QoL aspects in HNC patients treated with radiotherapy and/or chemotherapy may require up to two years. Prolonged monitoring and management of oral symptoms could enhance QoL. Future research should extend follow-up beyond two years for comprehensive interventions enhancing patient QoL.
Keywords: Head and neck cancer, Radiotherapy, Chemotherapy, Concurrent chemoradiotherapy, Quality of life
Introduction
Head and neck cancer (HNC) can involve multiple sites, including the oral cavity, pharynx, larynx, nose, sinuses, salivary glands, thyroid gland, and ears,1 and accounts for approximately 4.7% (or approximately 27,700) of all cancers in Japan.2 According to a report from the Japan Society for HNC,3 the number of patients on its HNC registry, which excludes thyroid cancer, has increased dramatically from 3203 in 2011 to 13,685 in 2019. However, in Japan, public awareness of HNC is lower than that of the five most prevalent cancers (lung, stomach, liver, colon, and breast), and HNC is not easily detected during a medical examination.4 Early detection of HNC is particularly difficult in Japan because of the lack of a government-funded screening program and a shortage of otolaryngologists and head/neck surgeons.
The head and neck area contains multiple organs that have important roles in the basic functions of daily life, including breathing, vocalization, articulation, mastication, and swallowing, and are important determinants of appearance. According to the 2022 Japanese Clinical Practice Guidelines for HNC,5 the mainstays of curative treatment for HNC are surgery and radiotherapy, with chemoradiotherapy (CRT) used to preserve function and morphology, treat locally advanced cases, and/or prevent recurrence in high-risk cases. The head and neck region also encompasses a wide range of anatomic sites, and treatment is selected based on comprehensive judgment of tumor histology, disease stage, indications for radical surgical resection, desire to preserve organ function, age, underlying disease, systemic function, and performance status, taking into account survival rates and organ dysfunction at the primary site.6 A meta-analysis by Pignon et al7 found evidence of a favorable outcome and an improved survival rate in patients with HNC who received CRT, namely, the addition of chemotherapy to local curative radiotherapy treatment, as a multidisciplinary strategy for advanced HNC, and demonstrated the usefulness of CRT as a treatment that combines local control with preservation of organ function and morphology. However, CRT has not been shown to improve the survival rate in patients older than 71 years,7 and problems such as deterioration of general health status and poor nutrition because of decreased oral intake as a result of the adverse effects of radiotherapy and/or chemotherapy have been reported.8 It is now clear that adverse events caused by radiotherapy and/or chemotherapy in patients with HNC can persist for several years after the end of treatment and decrease quality of life (QoL).9,10 Therefore, attenuation of the adverse effects of radiotherapy and/or chemotherapy and improvement of post-treatment QoL are important for cancer survivors.
Several previous studies have evaluated QoL as an outcome when comparing the effectiveness and safety of surgical treatment with that of conservative treatment, but over relatively short-term follow-up durations.11, 12, 13, 14 Late adverse events after radiotherapy and/or chemotherapy for HNC that affect QoL have not been clearly defined in terms of content and timing, and few studies have included more than 1 year of follow-up, particularly in Japan.13, 14, 15, 16 Better long-term management of patients who receive radiotherapy and/or chemotherapy for HNC requires evidence from longitudinal studies that focus on the time course of QoL in these patients in the longer term. The purpose of this study was to clarify the time course of patients’ QoL in the 2 years following radiotherapy and/or chemotherapy for HNC.
Methods
Study design
The study had a prospective, longitudinal observational design.
Patients
Patients with a diagnosis of HNC who were admitted to Fukuoka University Hospital between January 2015 and January 2017 to receive radiotherapy only, chemotherapy only, or concurrent CRT as curative or post-recurrence treatment were eligible for this study. The following exclusion criteria were applied: age younger than 20 years; surgical treatment already planned on admission; inability to provide informed consent; auditory or visual impairment that led to difficulty reading or listening to explanations of the questionnaire; and difficulty answering the questionnaire for cognitive or other reasons. All patients who satisfied the inclusion/exclusion criteria were invited to participate in the study.
Data collection
QoL and social background data were obtained from responses to questionnaires completed on admission (pre-treatment), before discharge (post-treatment), and 3, 6, and 12 months and 2 years after discharge. Information on diagnosis, tumor site, cancer stage, treatment, lifestyle factors, and disease progression during the follow-up period was obtained from the electronic medical records. Patients were censored in the event of death, withdrawal of consent, or loss of follow-up.
QoL questionnaires
QoL was measured using three instruments: (1) the Short-Form 36-Item Health Survey version 2 (SF36v2®), which is a comprehensive QoL measure that allows comparison with healthy national norms; (2) the European Organization for Research and Treatment of Cancer quality of life (EORTC-QLQ-C30) questionnaire, which is an integrated system for assessing health-related QoL in patients with cancer; and (3) the European Organization for Research and Treatment of Cancer quality of life head and neck-35 (EORTC-QLQ-H&N35) questionnaire, which is designed for use in combination with the EORTC-QLQ-C30 in patients with HNC.
Short-form 36-item health survey version 2
The SF36v2 was created in the US and has been used internationally after being examined from the concept construction stage through psychometric evaluation. The Japanese version of the SF36 was developed by Fukuhara et al,17,18 and its reliability and validity have been established. The SF36 is built on a universal concept of health and includes 35 questions that measure eight health concepts and one that measures changes in health. The subscales of the eight concepts are physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health. Respondents are asked to rate each of these items using five or six options. Scores are calculated according to the standard SF36 program recommended by the health evaluation organization. Furthermore, by using a regression equation with weighting for each of the subscales, it is possible to obtain a summary score that includes three components: a physical component summary, which represents the physical aspects of QoL; a mental component summary, which represents the mental aspects of QoL; and a role-social component score, which represents the role/social health QoL specific to Japan.19 Subscales and summary scores are obtained by calculation of the norm-based score (NBS), where the NBS is 50 points and the standard deviation is 10 points. NBS values above or below 50 can be interpreted as being above or below the national norm. Registered for use with Hope International, the standard Japanese version of the SF36 (with a 1-month look-back period) was used at admission and at 3, 6, and 12 months and 2 years after discharge, while the acute Japanese version of the SF36 (with a 1-week look-back period) was used at the time of discharge in this study.
EORTC-QLQ-C30 and EORTC-QLQ-H&N35
The European Organization for Research and Treatment of Cancer (EORTC) developed the EORTC-QLQ-C30 and EORTC-QLQ-H&N35 questionnaires to measure QoL in patients with cancer. These two questionnaires are recommended to be used as a set.20 The Japanese version of the EORTC-QLQ-C30 was developed by Kobayashi et al,21 and its reliability and validity have been established. Global health status/QoL has 2 items and functional scales consisting of 15 items (physical functioning, n = 5; role functioning, n = 2; emotional functioning, n = 2; cognitive functioning, n = 4; and social functioning, n = 2). The symptom scale consists of 13 items (fatigue, n = 3; nausea, vomiting, and pain, n = 2 each; and dyspnea, insomnia, anorexia, constipation, diarrhea, and economic difficulties, n = 1 each). Global health status is rated using 7 response options, and the functional and symptom scales are rated using 4 response options. The Japanese version of the EORTC-QLQ-H&N35 was developed by Toth et al,22 and its reliability and validity have been confirmed. Symptom scales include 13 items (pain, swallowing, senses problems, speech problems, trouble with social eating, trouble with social contact, diminished libido, teeth, opening mouth, dry mouth, sticky saliva, coughing, and feeling ill), each with 4 response options, and 5 additional items (use of pain medication, nutritional supplements, feeding tube, weight loss, and weight gain), each with 2 response options.
Higher global health status and functional scale scores indicate better QoL. The higher the symptom scale score, the more severe the subjective symptoms. We registered with the EORTC and downloaded the Japanese version of the questionnaire for use in this study.
Date analysis
The responses to the SF36, EORTC-QLQ-C30, and EORTC-QLQ-H&N35 were examined using repeated measures analysis of variance. Tukey's test was used to compare values obtained before discharge with pre-treatment values and those obtained at 3, 6, and 12 months and 2 years after discharge. At each time point, missing QoL scores (for participants who died, withdrew their consent, were lost to follow-up, or did not respond) were not imputed. A stratified analysis of the time course of QoL scores was performed by treatment category. All statistical analyses were performed using JMP version 14-3 statistical discovery software (SAS Institute Inc., Cary, NC, USA). A P-value < 0.05 was considered statistically significant.
Ethical considerations
The study was approved by the Fukuoka University Medical Ethics Review Board (IRB No. 14-10-04). Written informed consent was obtained from all study participants after they had received a written and verbal explanation of the study.
Results
Patients
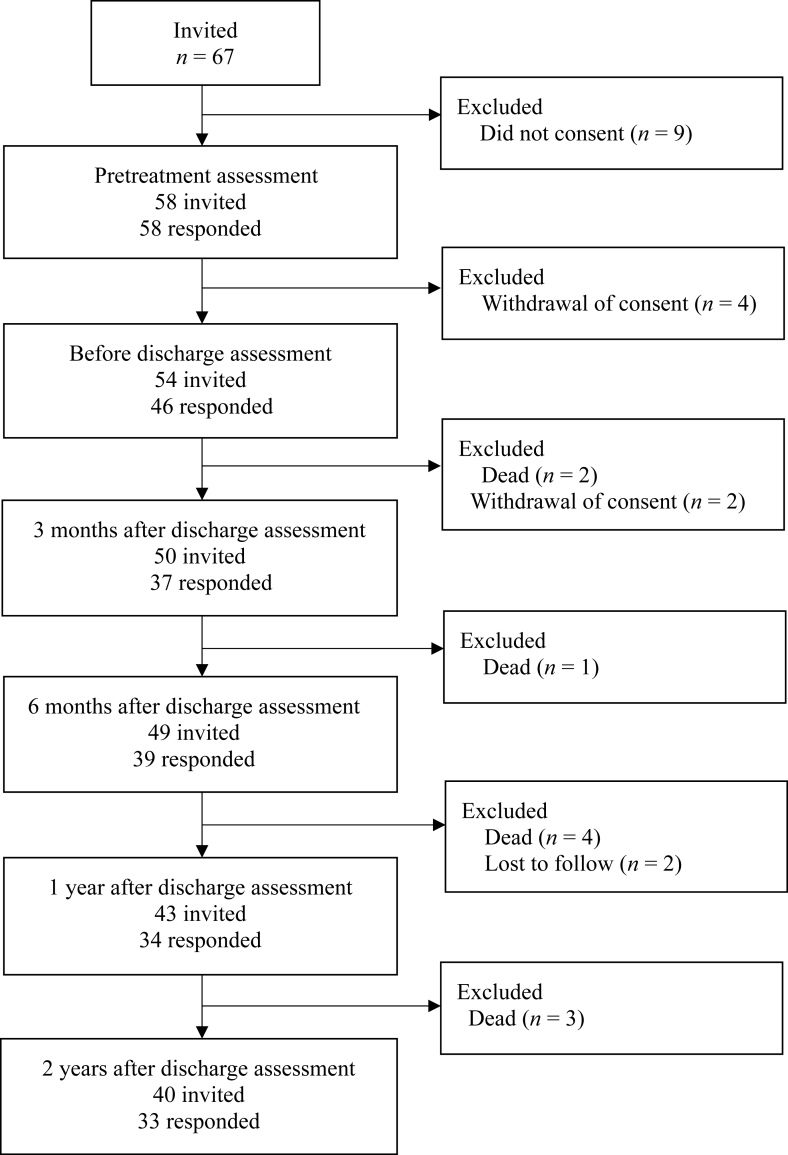
The patient selection process is shown in Fig. 1. Sixty-seven patients who met the study eligibility criteria were invited to participate in the study, 58 of whom consented to enrollment and completed all assessments before treatment. Four patients withdrew consent before discharge, leaving 54 patients, 46 of whom completed all assessments at the time of discharge. Two patients died and a further 2 withdrew consent during the first 3 months after discharge, leaving 50 patients, 37 of whom completed all assessments at 3 months. One patient died between 3 and 6 months after discharge, leaving 49 participants, 39 of whom completed assessments at 6 months. Four further patients died and 2 were lost to follow-up between 6 months and 1 year after discharge, leaving 43 participants, 34 of whom completed assessments at 12 months. After the exclusion of 3 patients who died between 1 and 2 years after discharge, 33 of 40 participants completed assessments at 2 years after discharge. Ten patients (17.2%) died during the 2-year follow-up period, and 8 (13.8%) withdrew their consent or were lost to follow-up. The mean follow-up duration was 1.3 years.
Fig. 1.
Flow chart showing the patient selection process.
The mean patient age was 65.2 ± 10.7 years (range 40 to 84) at baseline (Table 1). Forty-seven patients (81.0%) were male, and 11 (19.0%) were female. Eleven patients (19.0%) lived alone, and 47 (81.0%) lived with two or more family members. Twenty-five (43.1%) of the respondents were employed. Twenty-two patients (37.9%) were current smokers, and 24 (41.4%) were ex-smokers. Thirty-three participants (56.9%) were current consumers of alcohol at baseline. Body mass index, calculated as body weight divided by the square of height, was normal at baseline (18.5–24.9) in 42 patients (72.4%). The most common tumor sites were the larynx (25.9%, n = 15) and hypopharynx (25.9%, n = 15), followed by the oropharynx (19.0%, n = 11), nasopharynx (8.6%, n = 5), and nasal or paranasal sinuses (6.9%, n = 4). The most common cancer stage was IV (67.2%, n = 39), followed by II (17.2%, n = 10) and III (15.5%, n = 9). Eight patients (13.8%) received radiotherapy only, 2 (3.4%) received chemotherapy only, and 48 (82.8%) received concurrent CRT.
Table 1.
Characteristics of the participants.
| Variables | Participants (n = 58) | |
|---|---|---|
| Age, yr, mean ± SD (range) | 65.2 ± 10.7 (40–84) | |
| Gender, n (%) | Male | 47 (81.0) |
| Female | 11 (19.0) | |
| No. of family members, n (%) | 1 | 11 (19.0) |
| 2 | 23 (39.7) | |
| ≥ 3 | 24 (41.4) | |
| Marital status, n (%) | Married | 41 (70.7) |
| Single or widowed | 16 (27.6) | |
| Other | 1 (1.7) | |
| Employment status, n (%) | Employed | 25 (43.1) |
| Unemployed | 33 (56.9) | |
| Educational background, n (%) | Junior high school graduate | 11 (19.0) |
| High school graduate | 25 (43.1) | |
| College graduate | 17 (29.3) | |
| Others | 5 (8.6) | |
| Smoking, n (%) | Daily smoker | 22 (37.9) |
| Non-smoker | ||
| Ex-smoker | 24 (41.4) | |
| Never smoker | 12 (20.7) | |
| Drinking, n (%) | Drinker | 33 (56.9) |
| Social drinker | 5 (8.6) | |
| Past drinker | 8 (13.8) | |
| Non-drinker | 12 (20.7) | |
| Body mass index, kg/m2, n (%) | ≥ 25 (obese) | 12 (20.7) |
| 18.5–25.0 (normal) | 42 (72.4) | |
| < 18.5 (underweight) | 4 (6.9) | |
| Tumor site, n (%) | Lip or oral cavity | 2 (3.4) |
| Nasal cavity or accessory sinuses | 4 (6.9) | |
| Nasopharynx | 5 (8.6) | |
| Oropharynx | 11 (19.0) | |
| Hypopharynx | 15 (25.9) | |
| Larynx | 15 (25.9) | |
| Major salivary gland | 3 (5.2) | |
| Thyroid | 1 (1.7) | |
| External ear | 2 (3.4) | |
| Cancer stage, n (%) | II | 10 (17.2) |
| III | 9 (15.5) | |
| IV | 39 (67.2) | |
| Treatment category, n (%) | Radiotherapy | 8 (13.8) |
| Chemotherapy | 2 (3.4) | |
| Concurrent chemoradiotherapy | 48 (82.8) | |
| Radiation dose, Gy, mean ± SD | 61.1 ± 13.8 | |
SF36 scores
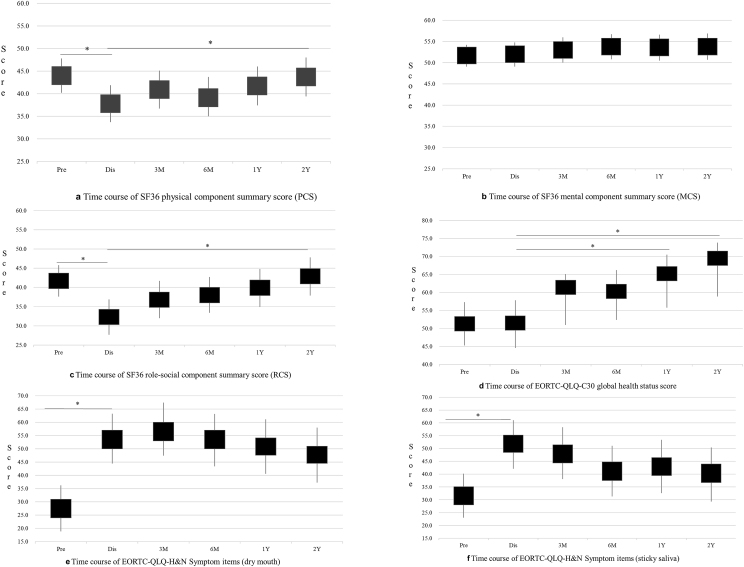
The physical and role-social component summary scores were lower than the NBS at all times, showing the greatest decrease before discharge and having recovered by 2 years after discharge (Table 2, Fig. 2a and c). The mental component summary score was slightly higher than the NBS at all time points and did not differ significantly between pre-treatment and 2 years after discharge (Fig. 2b). In the subscales, all scores were lowest before discharge, especially physical functioning, role-physical functioning, and vitality, which were significantly worse at the end of treatment. Mental health had recovered by 6 months after discharge; physical functioning, social functioning, and role-emotional functioning had recovered by 1 year after discharge, and physical functioning, bodily pain, and vitality had recovered by 2 years after discharge. There was no significant difference in general health between pre-treatment and 2 years after discharge (Table 2).
Table 2.
Time course of SF36 scores.
| Parameter | Pretreatment (n = 58) | Before discharge (n = 46) | 3 months after discharge (n = 37) | 6 months after discharge (n = 39) | 1 year after discharge (n = 34) | 2 years after discharge (n = 33) |
|---|---|---|---|---|---|---|
| Summary score | ||||||
| Physical component summary | 44.0 (40.2–47.8)∗ | 37.8 (33.7–41.9) | 40.9 (36.7–45.1) | 39.1 (35.0–43.7) | 41.7 (37.4–46.0) | 43.7 (39.4–48.0)∗ |
| Mental component summary | 51.7 (49.1–54.2) | 52.0 (49.1–54.8) | 53.0 (50.0–56.0) | 53.8 (50.8–56.7) | 53.6 (50.5–56.6) | 53.8 (50.7–56.9) |
| Role-social component summary | 41.7 (37.6–45.8)∗ | 32.3 (27.7–36.9) | 36.8 (32.0–41.7) | 38.0 (33.4–42.7) | 39.9 (34.9–44.8) | 42.9 (37.9–47.8)∗ |
| Sub scales | ||||||
| Physical functioning | 78.9 (72.2–85.5)∗ | 64.4 (57.3–71.4) | 71.4 (64.0–78.8) | 69.9 (62.6–77.1) | 74.3 (66.7–81.8)∗ | 77.3 (69.7–84.8)∗ |
| Role Physical | 69.5 (61.6–77.4)∗ | 52.1 (43.3–60.8) | 62.1 (52.9–71.2) | 61.3 (52.3–70.3) | 64.2 (54.8–73.8) | 73.9 (64.4–83.4)∗ |
| Bodily pain | 64.8 (58.0–71.6) | 59.6 (52.1–67.1) | 67.8 (59.8–75.9) | 67.2 (59.3–75.1) | 72.3 (64.0–80.7) | 74.8 (66.3–83.2)∗ |
| Social functioning | 70.3 (63.0–77.5) | 59.1 (51.0–67.3) | 68.0 (59.4–76.7) | 70.1 (61.6–78.5) | 73.6 (64.7–82.5)∗ | 76.8 (67.8–85.7)∗ |
| General health perceptions | 52.1 (47.2–57.0) | 49.7 (44.4–54.9) | 53.7 (48.1–59.3) | 52.5 (47.1–58.0) | 54.8 (49.1–60.4) | 57.3 (51.6–62.9) |
| Vitality | 62.5 (56.5–68.5)∗ | 50.4 (43.9–57.0) | 56.4 (49.4–63.3) | 56.9 (50.1–63.8) | 58.4 (51.3–65.6) | 63.8 (56.5–71.0)∗ |
| Role emotional | 67.7 (59.8–75.5) | 55.0 (46.1–63.9) | 63.3 (54.1–72.6) | 64.8 (55.7–73.9) | 70.4 (60.8–78.0)∗ | 76.3 (66.7–85.9)∗ |
| Mental health | 66.7 (61.5–71.8) | 60.9 (55.3–66.5) | 65.9 (60.0–71.8) | 69.6 (63.8–75.4)∗ | 69.3 (63.2–75.4) | 72.6 (66.4–78.7)∗ |
Values are shown as the mean (95% confidence interval), ∗P < 0.05 vs. before discharge.
Fig. 2.
Time course of QOL score. Pre, Pretreatment; Dis, Before discharge; 3M, 3 months after discharge; 6M, 6 months after discharge; 1Y, 1 year after discharge; 2Y, 2 years after discharge. Black boxes represent mean value. Vertical lines represent 95% confidence intervals. ∗P <0.05 vs. before discharge.
EORTC-QLQ-C30 scores
According to the EORTC-QLQ-C30 scores, there was no significant difference in global health status/QoL between pre-treatment and before discharge; however, there was a significant improvement at 1 year after discharge (Table 3, Fig. 2d). Physical functioning and social functioning did not differ between pre-treatment and discharge but had improved significantly by 2 years and 3 months, respectively, after discharge. There was no significant change in emotional or cognitive functioning in the 2 years after discharge. Symptom scales showed that loss of appetite and constipation were the worst symptoms before treatment; appetite improved during the 2 years following discharge, and constipation improved by 3 months after discharge. Pain was worst before discharge but had improved significantly by 1 year after discharge. Financial difficulties were also worst before treatment but had improved significantly by 3 months following discharge (Table 3).
Table 3.
Time course of EORTC-QLQ-C30 scores.
| Parameter | Pretreatment (n = 58) | Before discharge (n = 46) | 3months after discharge (n = 37) | 6months after discharge (n = 39) | 1year after discharge (n = 34) | 2years after discharge (n = 33) |
|---|---|---|---|---|---|---|
| Global health status/QoL | 51.3 (45.3–57.3) | 51.5 (44.6–57.8) | 61.4 (51.0–65.1) | 60.3 (52.4–66.2) | 65.2 (55.8–70.5)∗ | 69.5 (58.9–73.8)∗ |
| Functional scales | ||||||
| Physical functioning | 81.8 (75.8–87.8)∗ | 66.8 (60.5–73.2) | 75.1 (68.4–81.8) | 75.2 (68.6–81.7) | 78.2 (71.4–85.0)∗ | 80.5 (73.7–87.4)∗ |
| Role functioning | 62.1 (54.7–69.5) | 64.5 (56.3–72.8) | 74.4 (62.6–80.2) | 74.8 (66.2–83.4) | 76.2 (67.0–85.4) | 82.4 (73.1–91.6)∗ |
| Emotional functioning | 77.0 (72.2–81.9) | 77.4 (72.2–82.6) | 80.9 (75.3–86.4) | 82.3 (76.9–87.7) | 81.4 (75.8–87.1) | 84.6 (78.9–90.2) |
| Cognitive functioning | 77.9 (73.0–82.8) | 72.4 (67.0–77.9) | 75.1 (69.3–80.9) | 76.1 (70.4–81.8) | 75.5 (69.5–81.6) | 75.4 (69.3–81.4) |
| Social functioning | 65.5 (59.5–71.5) | 67.0 (60.3–73.8) | 81.2 (74.1–88.6)∗ | 81.6 (74.6–88.6)∗ | 86.6 (79.2–94.2)∗ | 85.7 (78.2–93.2)∗ |
| Symptom scales | ||||||
| Fatigue | 31.1 (25.1–37.1) | 46.4 (39.7–53.2) | 41.3 (34.3–48.4) | 41.1 (34.1–48.0) | 38.0 (30.6–45.4) | 36.6 (29.2–44.0) |
| Nausea and vomiting | 3.2 (0.5–5.8) | 8.6 (5.6–11.6) | 4.1 (0.8–7.4) | 4.4 (1.3–7.6) | 2.8 (−0.7-6.2) | 3.1 (−0.4-6.5) |
| Pain | 25.4 (19.1–31.8) | 34.4 (27.2–41.5) | 20.0 (14.4–29.6) | 22.0 (15.4–30.3) | 18.6 (10.6–26.6)∗ | 19.6 (11.7–27.6) ∗ |
| Dyspnea | 19.5 (13.0–26.1) | 26.7 (19.4–34.0) | 29.1 (21.4–36.8) | 25.2 (17.7–32.8) | 23.5 (15.5–31.5) | 23.9 (15.8–32.0) |
| Insomnia | 21.8 (15.3–28.4) | 27.9 (20.6–35.3) | 24.6 (16.8–32.4) | 24.5 (16.8–32.1) | 27.6 (19.4–35.8) | 21.9 (13.7–30.1) |
| Appetite loss | 16.7 (9.3–24.0)∗ | 40.3 (32.0–48.5) | 33.2 (24.5–42.0) | 31.2 (24.6–41.7) | 27.0 (17.9–36.2) | 23.1 (13.9–32.2)∗ |
| Constipation | 20.7 (14.0–27.4)∗ | 34.0 (26.6–41.4) | 20.0 (11.6–27.8)∗ | 18.3 (10.4–26.2)∗ | 24.7 (16.3–33.2) | 21.7 (13.1–30.1) |
| Diarrhea | 10.9 (6.2–15.6) | 12.0 (6.6–17.4) | 10.7 (4.9–16.5) | 8.9 (3.2–14.6) | 13.3 (7.1–19.4) | 17.5 (11.4–23.6) |
| Financial difficulties | 35.1 (28.1–42.0) | 30.6 (22.9–38.3) | 16.3 (8.2–24.5)∗ | 22.3 (14.3–30.3) | 19.1 (10.7–27.6) | 16.8 (8.3–25.2)∗ |
Values are shown as the mean (95% confidence interval), ∗P < 0.05 vs before discharge.
EORTC-QLQ-H&N35 scores
Scores for swallowing, senses problems, trouble with social eating, trouble with social contact, dry mouth, sticky saliva, coughing, and feeding tube were significantly worse before discharge; pain killers, feeding tube, and weight loss had recovered significantly by 3 months after discharge; trouble with social contact by 1 year after discharge; and pain, swallowing, senses problems, trouble with social eating, and coughing by 2 years after discharge. There was no improvement in dry mouth or sticky saliva at 2 years after discharge (Table 4, Fig. 2e and f).
Table 4.
Time course of EORTC-QLQ-H&N35 scores.
| Parameter | Pretreatment (n = 58) | Before discharge (n = 46) | 3 months after discharge (n = 37) | 6 months after discharge (n = 39) | 1 year after discharge (n = 34) | 2 years after discharge (n = 33) |
|---|---|---|---|---|---|---|
| Symptom items | ||||||
| Pain | 16.7 (11.7–21.6) | 23.4 (17.9–29.0) | 17.8 (11.9–23.7) | 16.4 (10.6–22.1) | 15.9 (9.8–22.0) | 9.1 (3.0–15.3)∗ |
| Swallowing | 22.1 (15.5–28.6)∗ | 35.2 (28.0–42.4) | 27.8 (20.4–35.3) | 27.5 (20.2–34.8) | 26.0 (18.3–33.8) | 23.2 (15.5–31.0)∗ |
| Senses problems | 13.5 (6.0–21.0)∗ | 36.5 (28.1–44.9) | 33.7 (24.9–42.5) | 32.0 (23.3–40.6) | 28.0 (18.9–37.2) | 19.0 (9.9–28.2)∗ |
| Speech problems | 31.0 (19.7–42.4) | 38.5 (25.6–51.4) | 29.5 (15.7–43.3) | 29.9 (16.4–43.3) | 30.9 (16.6–45.2) | 42.6 (28.2–57.1) |
| Trouble with social eating | 24.3 (19.0–29.7)∗ | 38.3 (32.4–44.2) | 30.9 (24.7–37.1) | 31.1 (25.0–37.2) | 28.7 (22.2–35.1) | 25.6 (19.2–32.1)∗ |
| Trouble with social contact | 17.8 (12.0–23.6)∗ | 29.9 (23.3–36.4) | 24.5 (17.7–31.1) | 19.9 (13.3–26.4) | 18.4 (11.4–25.3)∗ | 14.2 (7.3–21.1)∗ |
| Less sexuality | 35.4 (25.5–45.4) | 40.9 (30.0–51.9) | 43.7 (32.4–55.0) | 44.6 (33.6–55.7) | 42.3 (30.6–53.9) | 51.4 (39.7–63.1) |
| Teeth | 14.4 (7.5–21.2) | 16.9 (9.2–24.5) | 23.4 (15.4–31.4) | 16.6 (8.8–24.4) | 17.5 (9.2–25.8) | 19.0 (10.7–27.4) |
| Opening mouth | 16.6 (9.2–24.2) | 19.5 (11.4–27.7) | 21.0 (12.4–29.6) | 18.7 (10.2–27.1) | 21.9 (13.0–30.8) | 16.2 (7.2–25.2) |
| Dry mouth | 27.6 (18.9–36.2)∗ | 53.8 (44.4–63.2) | 57.4 (47.4–67.4) | 53.3 (43.4–63.1) | 50.8 (40.6–61.1) | 47.7 (37.3–58.0) |
| Sticky saliva | 31.6 (23.0–40.2)∗ | 51.6 (42.1–61.1) | 48.2 (38.1–58.3) | 41.2 (31.3–51.1) | 43.0 (32.6–53.4) | 39.9 (29.3–50.4) |
| Coughing | 22.4 (14.9–29.9)∗ | 39.8 (31.5–48.1) | 31.7 (23.0–40.5) | 33.3 (24.7–41.9) | 31.2 (22.2–40.3) | 23.6 (14.5–32.7)∗ |
| Felt ill | 32.7 (23.4–42.1) | 41.2 (30.8–52.0) | 38.4 (27.1–49.8) | 32.5 (21.4–43.6) | 29.7 (18.0–41.5) | 28.5 (16.6–40.4) |
| Additional items | ||||||
| Pain killers | 36.2 (25.0–47.4) | 54.9 (42.1–67.7) | 28.6 (14.9–42.3)∗ | 15.3 (1.9–28.7)∗ | 18.5 (4.3–32.7)∗ | 10.7 (−3.7-25.0)∗ |
| Nutritional supplements | 22.4 (10.7–34.2) | 37.1 (23.8–50.5) | 30.7 (16.4–45.0) | 23.7 (9.7–37.6) | 35.9 (21.1–50.7) | 26.0 (11.0–40.9) |
| Feeding tube | 3.4 (−1.7-8.6)∗ | 14.1 (8.2–20.0) | 1.4 (−5.0-7.7)∗ | 3.0 (−3.2-9.2) | 3.8 (−2.8-10.4) | 0.4 (−6.3-7.0)∗ |
| Weight loss | 41.4 (29.6–53.2) | 52.3 (38.4–66.1) | 25.2 (10.7–39.8)∗ | 20.4 (6.2–34.6)∗ | 21.9 (6.8–37.0)∗ | 24.0 (9.4–40.0)∗ |
| Weight gain | 12.3 (1.5–23.2) | 14.8 (2.2–27.4) | 37.8 (24.4–51.3) | 28.7 (15.7–41.8) | 20.5 (6.5–34.5) | 33.4 (19.2–47.6) |
Values are shown as the mean (95% confidence interval), ∗P < 0.05 vs before discharge.
Time course of QoL scores by treatment category
Supplementary Table S1 shows the time course of QoL scores by treatment category. The time course of these scores was similar for the 3 groups, although the numbers of patients who received radiotherapy only (n = 8) and those who received chemotherapy only (n = 2) were small.
Discussion
Summary of results
In this long-term observational study, some aspects of QoL (ie, the physical and role-social components of the SF36 and EORTC-QLQ-C30) gradually recovered during the 2 years following completion of CRT in patients with HNC. Furthermore, some symptoms, such as dry mouth and viscosity of saliva, had not resolved even by 2 years after completion of treatment.
SF36
The SF36 has three component summary scores: physical, mental, and role social. With regard to the physical component, previous studies in patients with HNC found that the physical component summary score remained lower than the pre-treatment value at 6 weeks,23 3 months,24 and 6 months25 after treatment but had improved by 1 year after treatment.24 In the present study, the physical component summary score was lowest at discharge but recovered to the pre-treatment value over the course of 2 years. As in the previous studies, we found that the physical component summary score decreased during treatment and took 1–2 years to recover. The mental component summary score was reported to be significantly lower at 6 weeks after treatment in one study,23 and at 3 months in another,24 but had recovered by 6 months in a further study.25 However, in the present study, the mental component summary score remained stable between pre-treatment and 2 years after treatment. The reasons for the inconsistency between the findings of previous studies and those of our present analysis are not clear, but the discrepancy in the mental component summary score might be attributable to differences in study design and in the selection/characteristics of participants between studies. Global data on the role-social component of the SF36 are limited because this component tends to be focused on in Asia rather than in other countries. In the present analysis, the role-social component score decreased significantly before discharge but recovered by 2 years after treatment. Future research is required to validate this finding.
EORTC-QLQ-C30
The EORTC-QLQ-C30 includes global health status/QoL and both functional and symptom scales. Previous studies have found global health status/QoL to be low at baseline, before discharge,26,27 and at 1–3 months after treatment28,29 but to have recovered by 1 year after discharge.26,27 In our study, as in previous reports, global health status/QoL remained low between baseline and 6 months after discharge had recovered significantly by 1 year after discharge, with the best result observed at 2 years after discharge. The findings of the previous studies and our present analysis show that global health status/QoL recovers gradually over a very long period of 1–2 years.
EORTC-QLQ-H&N35
The EORTC-QLQ-H&N35 consists of 8 symptom scales. In previous studies, dry mouth and sticky saliva were worse at the end of treatment than before treatment30 with no recovery in dry mouth30, 31, 32 or sticky saliva30,32 even after 1 year. One study found that dry mouth and sticky saliva scores continued to be significantly worse at 3 years after the end of treatment for HNC than those in a sample from the general population.33 In our study, as in previous reports, dry mouth and sticky saliva were worst at the time of discharge after treatment and had not resolved 2 years later, suggesting that these symptoms are likely to persist in the long term. Exactly why some components of QoL recovered at 2 years despite persistent symptoms of dry mouth and sticky saliva is not clear, but one reason may be that local symptoms are compensated for by the recovery of other symptoms.
Limitations
The strength of this study is that QoL was monitored for a long period of time (ie, 2 years) in patients with various stages of HNC in Japan. We also used a variety of QoL scoring methods, including the SF36, EORTC-QLQ-C30, and EORTC-QLQ-H&N35, to ensure that subjective health status was evaluated as accurately as possible.34 However, our study also has some limitations. First, the sample size was small (n = 58). Second, the dropout rate during follow-up was 31.0%, which is somewhat high but inevitable because of death, withdrawal of consent because of the onset of dementia, or poor physical health status. Third, the study was performed at a single center, which means that the possibility of selection bias cannot be excluded.
Conclusions
This study investigated QoL after radiotherapy and/or chemotherapy in Japanese patients with HNC who were followed up for 2 years after discharge using the SF36v2, the EORTC-QLQ-C30, and the EORTC-QLQ-H&N35 questionnaires. Some components of QoL were worst at the time of discharge after treatment and took up to 2 years to recover. Long-term monitoring and management of oral symptoms might help improve QoL in patients who have undergone treatment for HNC. Future research on interventions to improve QoL in these patients might require follow-up assessments for more than 2 years.
Acknowledgments
We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
CRediT author statement
Kazuyo Iwanaga: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing Original and Revised draft preparation, Funding acquisition. Yoko Ishibashi, Kaori Maki and Ayako Ura: Investigation, Writing – Revised draft preparation. Kumiko Kotake, Kaori Haba: Writing – Revised draft preparation. Toshifumi Sakata, Takashi Nakagawa: Resources, Writing – Revised draft preparation. Hisatomi Arima: Formal analysis, Writing – Original and Revised draft preparation, Supervision. All authors had full access to all the data in the study, and the corresponding author had final responsibility for the decision to submit for publication. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Declaration of competing interest
The authors declare no conflict of interest.
Funding
The study was supported by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (C) (Grant No. 5463454). The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Ethics statement
This study was conducted after obtaining approval from the Fukuoka University Medical Ethics Review Board (IRB No. 14-10-04). All participants provided written informed consent.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, upon reasonable request.
Declaration of generative AI in scientific writing
No AI tools or services were used during the preparation of this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.apjon.2023.100301.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Japan Society for Head and Neck Cancer. http://www.jshnc.umin.ne.jp/general/about.html. Accessed 20 March 2023. [In Japanese].
- 2.Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan. https://ganjoho.jp/reg_stat/statistics/stat/cancer/index.html. Accessed 25 June 2022. [In Japanese].
- 3.Japan Society for Head and Neck Cancer . 2019. Report of Head and Neck Cancer Registry of Japan Clinical Statistics of Registered Patients.http://www.jshnc.umin.ne.jp/pdf/HNCreport_2019.pdf [In Japanese] [Google Scholar]
- 4.Yoshino K. [How to build head and neck cancer treatment in Japan - based on Osaka Prefecture's Cancer Treatment Cooperation System] Nippon Jibiinkoka Gakkai Kaiho. 2010;113:882–888. doi: 10.3950/jibiinkoka.113.882. In Japanese. [DOI] [PubMed] [Google Scholar]
- 5.Japan Society for Head and Neck Cancer . Japanese Clinical Practice Guidelines for Head and Neck Cancer 2022. Kanehara-Shuppan; Tokyo: 2022. [In Japanese] [Google Scholar]
- 6.Kobayashi T., Tahara M. [Advances in cancer chemotherapy. Details: cancer treatment by organ: head and neck cancer] Antibiotics & Chemotherapy. Special issue. 2011;27:1149–1157. [In Japanese] [Google Scholar]
- 7.Pignon J.P., le Maitre A., Maillard E., Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Fujii M. [New developments in chemoradiotherapy for head and neck cancer] J Jnp Soc Head Neck Surg. 2013;23:301–305. doi: 10.5106/jjshns.23.301. In Japanese. [DOI] [Google Scholar]
- 9.De Felice F., de Vincentiis M., Luzzi V., et al. Late radiation-associated dysphagia in head and neck cancer patients: evidence, research and management. Oral Oncol. 2018;77:125–130. doi: 10.1016/j.oraloncology.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Strojan P., Hutcheson K.A., Eisbruch A., et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat Rev. 2017;59:79–92. doi: 10.1016/j.ctrv.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.List M.A., Bilir S.P. Functional outcomes in head and neck cancer. Semin Radiat Oncol. 2004;14:178–189. doi: 10.1053/j.semradonc.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Christopher K.M., Osazuwa-Peters N., Dougherty R., et al. Impact of treatment modality on quality of life of head and neck cancer patients: findings from an academic medical institution. Am J Otolaryngol. 2017;38:168–173. doi: 10.1016/j.amjoto.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Aoki T., Ota Y., Suzuki T., et al. Longitudinal changes in the quality of life of oral cancer patients during the perioperative period. Int J Clin Oncol. 2018;23:1038–1045. doi: 10.1007/s10147-018-1322-3. [DOI] [PubMed] [Google Scholar]
- 14.Ebisumoto K., Okami K., Maki D., et al. Quality of life and swallowing function assessment before and after transoral resection for tonsillar cancer. Nippon Jibiinkoka Gakkai Kaiho. 2018;121:1071–1078. doi: 10.3950/jibiinkoka.121.1071. In Japanese. [DOI] [Google Scholar]
- 15.Shinozaki T., Ebihara M., Iwase S., et al. Quality of life and functional status of terminally ill head and neck cancer patients: a nation-wide, prospective observational study at tertiary cancer centers in Japan. Jpn J Clin Oncol. 2017;47:47–53. doi: 10.1093/jjco/hyw138. [DOI] [PubMed] [Google Scholar]
- 16.Harako C., Kurosawa M., Nishizawa Y. [A study on the QOL of patients with head and neck cancer] Journal of Health Sciences Research. 2013;3:13–22. https://hoken-kagaku.com/journal/Vol3(2013).pdf In Japanese. [Google Scholar]
- 17.Fukuhara S., Bito S., Green J., Hsiao A., Kurokawa K. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J Clin Epidemiol. 1998;51:1037–1044. doi: 10.1016/s0895-4356(98)00095-x. [DOI] [PubMed] [Google Scholar]
- 18.Fukuhara S., Ware J.E., Kosinski M., Wada S., Gandek B. Psychometric and clinical tests of validity of the Japanese SF-36 Health Survey. J Clin Epidemiol. 1998;51:1045–1053. doi: 10.1016/s0895-4356(98)00096-1. [DOI] [PubMed] [Google Scholar]
- 19.Suzukamo Y., Fukuhara S., Green J., Kosinski M., Ware J.E. Validation testing of a three-component model of Short Form-36 scores. J Clin Epidemiol. 2011;64:301–308. doi: 10.1016/j.jclinepi.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Bjordal K., de Graeff A., Fayers P.M., et al. A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. Eur J Cancer. 2000;36:1796–1807. doi: 10.1016/S0959-8049(00)00186-6. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi K., Takeda F., Teramukai S., et al. A cross validation of the European organization for research and treatment of cancer QLQ-C30 for Japanese with lung cancer. Eur J Cancer. 1998;34:810–815. doi: 10.1016/s0959-8049(97)00395-x. [DOI] [PubMed] [Google Scholar]
- 22.Toth G., Sakaguchi T., Mikami Y., Hirose H., Tsukuda M. A pilot study of the translation, cultural adaptation and validation of the EORTC head and neck cancer quality of life questionnaire module for use in Japan. Auris Nasus Larynx. 2005;32:175–183. doi: 10.1016/j.anl.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Samuel S.R., Maiya G.A., Babu A.S., Vidyasagar M.S. Effect of exercise training on functional capacity & quality of life in head & neck cancer patients receiving chemoradiotherapy. Indian J Med Res. 2013;137:515–520. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3705659/ [PMC free article] [PubMed] [Google Scholar]
- 24.Howren M.B., Christensen A.J., Hynds Karnell L., Van Liew J.R., Funk G.F. Influence of pretreatment social support on health-related quality of life in head and neck cancer survivors: results from a prospective study. Head Neck. 2013;35:779–787. doi: 10.1002/hed.23029. [DOI] [PubMed] [Google Scholar]
- 25.Funk G.F., Karnell L.H., Dawson C.J., et al. Baseline and post-treatment assessment of the general health status of head and neck cancer patients compared with United States population norms. Head Neck. 1997;19:675–683. doi: 10.1002/(sici)1097-0347(199712)19:8%3C675::aid-hed5%3E3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Oates J.E., Clark J.R., Read J., et al. Prospective evaluation of quality of life and nutrition before and after treatment for nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2007;133:533–540. doi: 10.1001/archotol.133.6.533. [DOI] [PubMed] [Google Scholar]
- 27.Kramer B., Wenzel A., Boerger M., et al. Long-term quality of life and nutritional status of patients with head and neck cancer. Nutr Cancer. 2019;71:424–437. doi: 10.1080/01635581.2018.1506492. [DOI] [PubMed] [Google Scholar]
- 28.do Nascimento Santos Lima E., Ferreira I.B., Lajolo P.P., Paiva C.E., de Paiva Maia Y.C., das Graças Pena G. Health-related quality of life became worse in short-term during treatment in head and neck cancer patients: a prospective study. Health Qual Life Outcome. 2020;18:307. doi: 10.1186/s12955-020-01543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Citak E., Tulek Z. Longitudinal quality of life in Turkish patients with head and neck cancer undergoing radiotherapy. Support Care Cancer. 2013;21:2171–2183. doi: 10.1007/s00520-013-1774-y. [DOI] [PubMed] [Google Scholar]
- 30.Tribius S., Raguse M., Voigt C., et al. Residual deficits in quality of life one year after intensity-modulated radiotherapy for patients with locally advanced head and neck cancer: results of a prospective study. Strahlenther Onkol. 2015;191:501–510. doi: 10.1007/s00066-015-0824-4. [DOI] [PubMed] [Google Scholar]
- 31.Bjordal K., Ahlner-Elmqvist M., Hammerlid E., et al. A prospective study of quality of life in head and neck cancer patients. Part II: longitudinal data. Laryngoscope. 2001;111:1440–1452. doi: 10.1097/00005537-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 32.Fang F.M., Chien C.Y., Kuo S.C., Chiu H.C., Wang C.J. Changes in quality of life of head-and-neck cancer patients following postoperative radiotherapy. Acta Oncol. 2004;43:571–578. doi: 10.1080/02841860410018430. [DOI] [PubMed] [Google Scholar]
- 33.Hammerlid E., Taft C. Health-related quality of life in long-term head and neck cancer survivors: a comparison with general population norms. Br J Cancer. 2001;84:149–156. doi: 10.1054/bjoc.2000.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohsawa I., Ishida T., Oshida Y., Yamanouchi K., Sato Y. Subjective health values of individuals with diabetes in Japan: comparison of utility values with the SF-36 scores. Diabetes Res Clin Pract. 2003;62:9–16. doi: 10.1016/S0168-8227(03)00145-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, upon reasonable request.